New Codex General Principles of Food Hygiene
Codex Alimentarius
The Codex Alimentarius Commission has now published a revised Code of Practice (General Principles of Food Hygiene (CXC 1-1969)) and its Annex Hazard Analysis and Critical Control Point (HACCP) System and Guidelines for its Application. The new 2020 revision covers General Principles of Food Hygiene: Good Hygiene Practices (GHPs) and the Hazard Analysis and Critical Control Point (HACCP) System.
According to the Codex Alimentarius Commission* the 2020 Revision of the General Principles of Food Hygiene (CXC 1-1969) and Hazard Analysis and Critical Control Point (HACCP) System and Guidelines for its Application this version of the code of practice: provides a common ground for the control of food safety worldwide and forms the basis for all other Codex hygiene texts and standards. The revision includes updates which will enable better application by food business operators, competent authorities and other stakeholders.
http://www.fao.org/fao-who-codexalimentarius/news-and-events/news-details/en/c/1310284/
The Codex Alimentarius, or “Food Code” is a collection of standards, guidelines and codes of practice adopted by the Codex Alimentarius Commission*. The Commission*, also known as CAC, is the central part of the Joint FAO/WHO Food Standards Programme and was established by FAO and WHO to protect consumer health and promote fair practices in food trade.
http://www.fao.org/fao-who-codexalimentarius/en/
Changes and the new Structure are Highlighted in the Table Below:

The major changes in CODEX HACCP 2020 are summarized in detail below:
- A new requirement for a Food Safety Culture
- New & Revised Definitions
- Enhanced Training Requirements
- Enhanced Good Hygiene System Requirements
- Enhanced Good Hygiene Practice Requirements in Control of Operations
- New Allergen Awareness/Management/Controls
- A new requirement for Product Traceability
- Enhanced Consumer Education Requirements
- Changes in HACCP Principles and a new requirement for Validation of the HACCP Plan
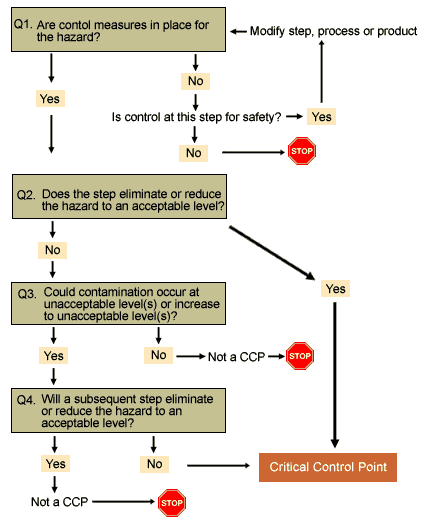
It is somewhat disappointing the “CODEX” Decision Tree has been removed and no alternative offered although Codex HACCP 2020 still makes reference to using a decision tree or other approach to identify critical control points (CCPs) in Chapter Two
Hazard Analysis and Critical Control Point (HACCP) System and Guidelines for its Application, Section 3: Application, 3.7 Determine the Critical Control Points (Step 7/ Principle 2)
The good news is that the Joint FAO/WHO Food Standards Programme Codex Alimentarius Commission 43rd Session 2020 Report (CAC Report 43) highlighted the following with regards to the Decision Tree:
Some Members from the LAC region, while supporting adoption and referring to written comments, highlighted:
- the importance of the decision tree for identifying critical control points (CCP) as it was essential for implementation of HACCP.
The Chairperson of CCFH clarified that work on the decision tree was currently at Step 2 of the procedure and would be considered at the next session. The decision tree is a useful tool for the application of the general principles document; and CAC43 adopted the revised GPFH at Step 5/8 and noted that work on the decision tree to identify critical control points (CCPs) will continue in CCFH so that once completed it could be included in the GPFH as an annex;
Codex HACCP 2020 Changes in more Detail
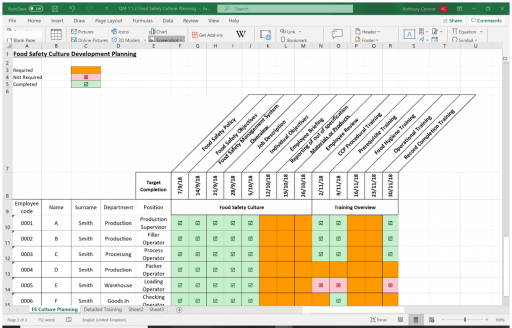
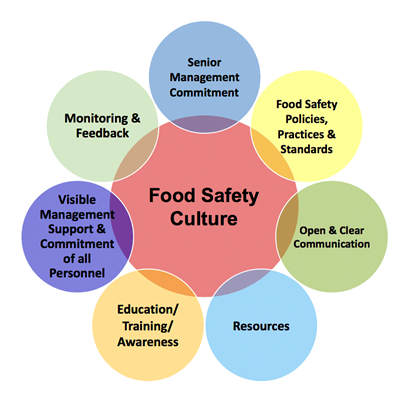
GENERAL PRINCIPLES – Management Commitment to Food Safety – Food Safety Culture
As part of Management Commitment to Food Safety the General Principles of Food Hygiene include a Food Safety Culture Requirement:
Fundamental to the successful functioning of any food hygiene system is the establishment and maintenance of a positive food safety culture acknowledging the importance of human behaviour in providing safe and suitable food.
Definitions
The two definitions sections in the previous version have been combined and extended. There are new definitions included for Acceptable level, Allergen cross-contact, Competent Authority, Contaminant, Contamination, Food business operator (FBO), Food Hygiene System, Good Hygiene Practices (GHPs), Prerequisite Programme and Significant hazard. Other changes are that Validation has been changed to a new definition Validation of control measures and the definition of Verification has been changed from the application of methods, procedures, tests and other evaluations, in addition to monitoring, to determine compliance with the HACCP plan to whether a control measure is or has been operating as intended.
Training
There is enhanced guidance for training to ensure that personnel have competence appropriate to the operations they are to perform.
RATIONALE: Training is fundamentally important to any food hygiene system and the competence of personnel.
There is guidance that: personnel tasked to perform any activities used in food control should be trained adequately to ensure that they are competent to perform their tasks and are aware of the impact of their tasks on the safety and suitability of the food. Systems should be in place to ensure that food handlers and personnel associated with the food business, such as maintenance staff, remain aware of all procedures necessary to maintain the safety and suitability of food. Records should be kept of training activities
Training programmes to be considered as appropriate to a person’s duties:
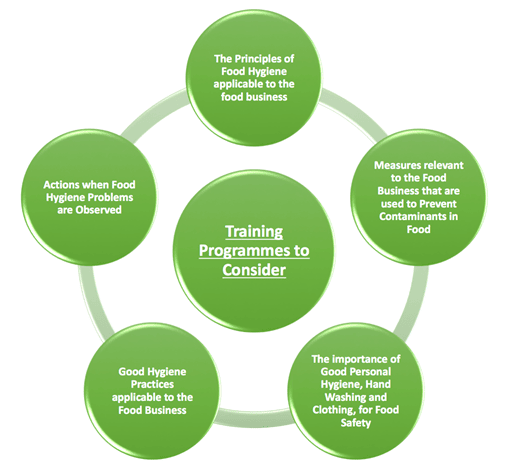
Elements to take into account in determining the extent of training required include: the use and maintenance of instruments and equipment associated with food.
In addition, for retail and food service operations, whether personnel have direct customer interaction is a factor in training, since it may be necessary to convey certain information about products (such as allergens) to customers.
Changes in Section 7: Control of Operation
Control of operation is achieved by having an appropriate food hygiene system in place. In section 7 there is new guidance for:
7.1 Description of products and processes
7.1.1 Product description
7.1.2 Process description
7.1.3 Consideration of the effectiveness of GHPs – When such increased attention on GHPs is insufficient to ensure food safety, it will be necessary to implement a HACCP system (Chapter 2).
7.1.4 Monitoring and corrective action
7.1.5 Verification
7.2 Key aspects of GHPs – Some key aspects of GHPs such as those described in Sections 7.2.1. and 7.2.2, could be considered as control measures applied at CCPs in the HACCP system.
7.2.5 Physical contamination
7.2.6 Chemical contamination
7.2.7 Allergen Management
CODEX 2020 states Systems for Allergen Awareness Control/Management should be in place including controls to prevent cross-contact.
Lot Identification & Traceability
The requirement for a traceability/product tracing system has been added in Codex HACCP 2020.
A traceability/product tracing system should be designed and implemented according to the Principles for Traceability/Product Tracing as a Tool within a Food Inspection and Certification System (CXG 60-2006), especially to enable the recall of the products, where necessary
Consumer Education
Consumer education guidance has been enhanced, programmes should enable consumers to understand the importance of any product label information and following any instructions accompanying products, and to make informed choices. In particular, consumers should be informed of the relationship between time/temperature control, cross contamination and foodborne illness, and of the presence of allergens.
Rather than an ANNEX, Hazard Analysis and Critical Control Point (HACCP) System and Guidelines for its Application is now Chapter 2

Changes in HACCP Principles
There have been some changes to the 7 HACCP Principles as per the diagram below:
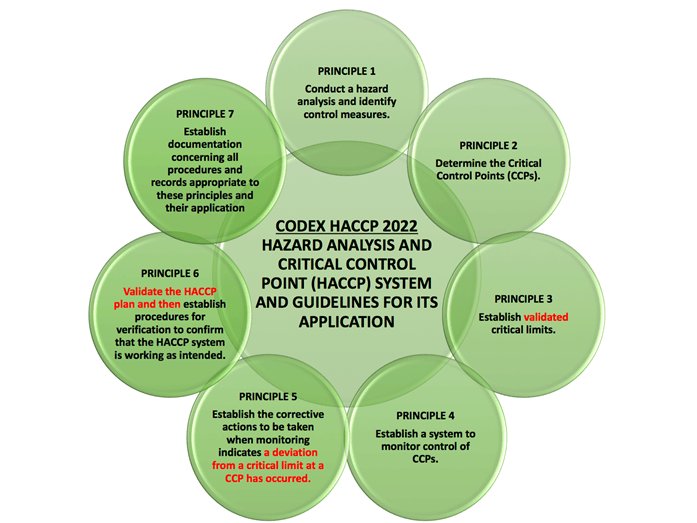
Validation of the HACCP Plan
There is an addition guidance 3.11.1 for Validation of the HACCP Plan:
Before the HACCP plan can be implemented, its validation is needed; this consists of making sure that the following elements together are capable of ensuring control of the significant hazards relevant to the food business: identifying the hazards, critical control points, critical limits, control measures, frequency and type of monitoring of CCPs, corrective actions, frequency and type of verification and the type of information to be recorded.
Chapter 2 includes a new ANNEX, Annex 1 – Comparison of control measures with examples.
Overall the changes are emphasizing the importance of using Good Hygiene Practices to Control Hazards in much the same way as the FSMA Final Rule for Preventive Controls for Human Food https://www.fda.gov/food/food-safety-modernization-act-fsma/fsma-final-rule-preventive-controls-human-food with a requirements for Monitoring, Corrective Action and Verification.
New Allergen Awareness, Management and Controls are more than overdue given the significant proportion of recalls worldwide that are due to incorrect labelling or allergen cross-contamination.