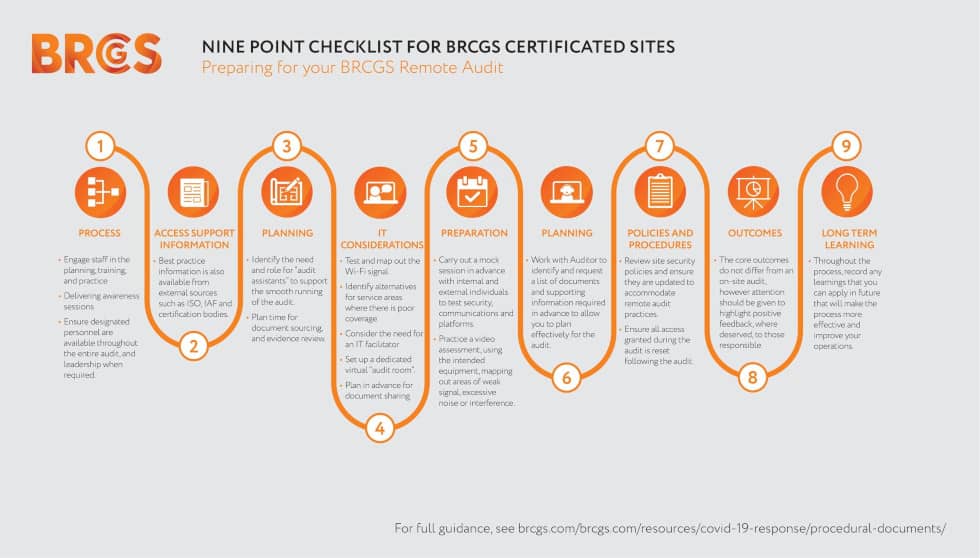
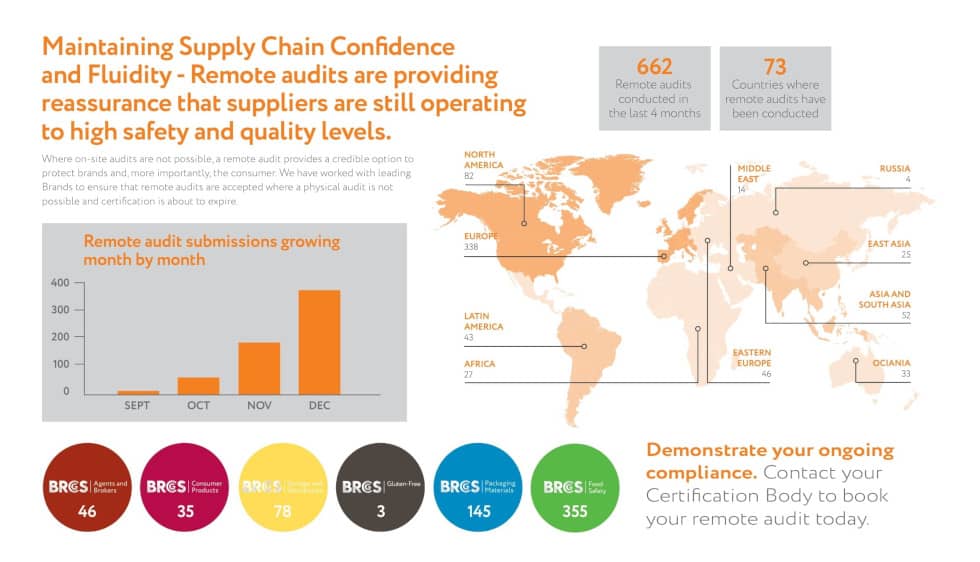
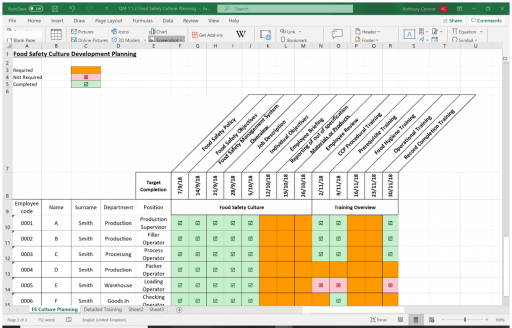
Integrating FSMA Requirements
In order to develop a Food Safety Management System compliant with both the BRCGS Global Standard and the FSMA you will first of all need to consider and comply with the requirements of the BRCGS Global Standard for Food Safety Issue 9 which requires compliance in 4 key areas; Senior Management Commitment, HACCP/Food Safety Plan, Product Safety and Quality Management System and Prerequisite Programs (which are covered in sections 4 to 9).
The BRCGS Global Standard for Food Safety Issue 9 requirements are in 9 Sections
1 Senior Management Commitment
2 The Food Safety Plan – HACCP
3 Food Safety and Quality Management System
4 Site Standards
5 Product Control
6 Process Control
7 Personnel
8 High-Risk, High-Care and Ambient High-Care Production Risk Zones
9 Requirements for Traded Products
For more information on compliance with BRCGS Global Standard for Food Safety Issue 9, see articles BRCGS Global Standard for Food Safety and What documents should be included in a BRCGS Global Standard for Food Safety Issue 9 compliant Food Safety Management System?
For sites that are legislated by 21 CFR Part 117 Preventive Controls for Human Food Rule, the requirements stipulated in Module 13: Meeting FSMA Requirements for Food must be considered in addition to those required by the BRCGS Global Standard for Food Safety Issue 9.
BRCGS Module 13: Meeting FSMA Requirements for Food outlines for food processors and manufacturers the requirements of the Food Safety Modernization Act (FSMA) Preventive Controls for Human Foods Rule that are not explicitly covered within the BRCGS Global Standard for Food Safety Issue 9.
For certification to Module 13, the following BRCGS Global Standard for Food Safety Issue 9 management clauses need to apply to the management of Module 13:
3.1 Food safety and quality manual
3.2 Documentation control
3.3 Record completion and maintenance
3.4 Internal audits
3.7 Corrective and preventive actions
3.8 Control of non-conforming product
3.10 Complaint handling
3.11 Management of incidents, product withdrawal and product recall
The areas not covered by the BRCGS standard specifically but required by the FSMA are in 5 categories:
Current good manufacturing practice, hazard analysis, and risk-based preventive controls for human food
Current good manufacturing practice and hazard analysis and risk-based preventive controls for food for animals
Mitigation strategies to protect food against intentional adulteration
Sanitary transportation of human and animal food
Standards for the growing, harvesting, packing, and holding of produce for human consumption
Current good manufacturing practice, hazard analysis, and risk-based preventive controls for human food areas that need to be addressed are described below.
Subpart B—Current Good Manufacturing Practice
(b) Plant construction and design
(3) Permit the taking of adequate precautions to protect food in installed outdoor bulk vessels by any effective means, including:
(5) Provide adequate lighting in hand-washing areas, dressing and locker rooms, and toilet rooms and in all areas where food is examined, manufactured, processed, packed, or held and where equipment or utensils are cleaned….
21 CFR § 117.37
(b) Plumbing. Plumbing must be of adequate size and design and adequately installed and maintained to:
(5) Provide that there is not backflow from, or cross-connection between, piping systems that discharge waste water or sewage and piping systems that carry water for food or food manufacturing.
- 117.40 Equipment and utensils
(a)
(4) Food-contact surfaces must be corrosion-resistant when in contact with food.
(6) Food-contact surfaces must be maintained to protect food from allergen crosscontact and from being contaminated by any source, including unlawful indirect food additives.
(b) Seams on food-contact surfaces must be smoothly bonded or maintained so as to minimize accumulation of food particles, dirt, and organic matter and thus minimize the opportunity for growth of microorganisms and allergen cross-contact
- 117.80 Processes and controls
(1) Raw materials and other ingredients must be inspected and segregated or otherwise handled as necessary to ascertain that they are clean and suitable for processing into food and must be stored under conditions that will protect against allergen cross-contact and against contamination and minimize deterioration. Raw materials must be washed or cleaned as necessary to remove soil or other contamination. Water used for washing, rinsing, or conveying food must be safe and of adequate sanitary quality. Water may be reused for washing, rinsing, or conveying food if it does not cause allergen cross-contact or increase the level of contamination of the food.
- 117.80 Processes and controls
(a) General.
(b) Raw materials and other ingredients.
(c) Manufacturing operations.
(9) Food, raw materials, and other ingredients that are adulterated: (i) Must be disposed of in a manner that protects against the contamination of other food; or (ii) If the adulterated food is capable of being reconditioned, it must be: (A) Reconditioned (if appropriate) using a method that has been proven to be effective; or (B) Reconditioned (if appropriate) and reexamined and subsequently found not to be adulterated within the meaning of the Federal Food, Drug, and Cosmetic Act before being incorporated into other food.
(16) When ice is used in contact with food, it must be made from water that is safe and of adequate sanitary quality in accordance with §117.37(a), and must be used only if it has been manufactured in accordance with current good manufacturing practice as outlined in this part.
- 117.95 Holding and distribution of human food by-products for use as animal food.
There are specific requirements for holding and distribution of human food by-products for use as animal food to be protected against contamination whilst also be identified and labelled.
- 117.110 Defect action levels.
There are requirements to reduce defects to minimum levels and prescribed limits for various commodities, see the Defect Levels Handbook, which is accessible at http://www.fda.gov/pchfrule and at https://www.fda.gov/food/ingredients-additives-gras-packaging-guidance-documents-regulatory-information/food-defect-levels-handbook#commodities
Subpart C—Hazard Analysis and Risk-Based Preventive Controls
- 117.126 Food safety plan.
(a) Requirement for a food safety plan.
(1) You must prepare, or have prepared, and implement a written food safety plan.
(2) The food safety plan must be prepared, or its preparation overseen, by one or more preventive controls qualified individuals.
(b) Contents of a food safety plan. The written food safety plan must include:
(1) The written hazard analysis as required by §117.130(a)(2);
(2) The written preventive controls as required by §117.135(b);
(3) The written supply-chain program as required by subpart G of this part;
(4) The written recall plan as required by §117.139(a); and
(5) The written procedures for monitoring the implementation of the preventive controls as required by §117.145(a)(1);
(6) The written corrective action procedures as required by §117.150(a)(1); and
(7) The written verification procedures as required by §117.165(b).
(a) Requirement for a hazard analysis.
(1) You must conduct a hazard analysis to identify and evaluate, based on experience, illness data, scientific reports, and other information, known or reasonably foreseeable hazards for each type of food manufactured, processed, packed, or held at your facility to determine whether there are any hazards requiring a preventive control.
(2) The hazard analysis must be written regardless of its outcome.
(b) Hazard identification. The hazard identification must consider:
(1) Known or reasonably foreseeable hazards that include:
(i) Biological hazards, including microbiological hazards such as parasites, environmental pathogens, and other pathogens;
(ii) Chemical hazards, including radiological hazards, substances such as pesticide and drug residues, natural toxins, decomposition, unapproved food or color additives, and food allergens; and
(iii) Physical hazards (such as stones, glass, and metal fragments); and
(2) Known or reasonably foreseeable hazards that may be present in the food for any of the following reasons:
(i) The hazard occurs naturally;
(ii) The hazard may be unintentionally introduced; or
(iii) The hazard may be intentionally introduced for purposes of economic gain.
- 117.135 Preventive controls.
(a)
(1) You must identify and implement preventive controls to provide assurances that any hazards requiring a preventive control will be significantly minimized or prevented ………
For food with a hazard requiring a preventive control:
(a) You must establish a written recall plan for the food.
- 117.140 Preventive control management components.
(a) Except as provided by paragraphs (b) and (c) of this section, the preventive controls required under §117.135 are subject to the following preventive control management components as appropriate to ensure the effectiveness of the preventive controls, taking into account the nature of the preventive control and its role in the facility’s food safety system:
(1) Monitoring in accordance with §117.145; – Establish monitoring activities and a written procedure for each preventive control
(2) Corrective actions and corrections in accordance with §117.150; – Establish corrective action procedures for when preventive controls are not in control and
(3) Verification in accordance with §117.155.
- 117.140 Preventive control management components.
(c) The recall plan established in §117.139 is not subject to the requirements of paragraph (a) of this section.
There are specific requirements and timescales for validation that the preventive controls identified and implemented in accordance with §117.135 are adequate to control the hazard as appropriate to the nature of the preventive control and its role in the facility’s food safety system.
- 117.165 Verification of implementation and effectiveness.
(a) Verification activities
Devices used to verify preventive controls must be calibrated
(4) Review of the following records within the specified timeframes, by (or under the oversight of) a preventive controls qualified individual,………….
(i) Records of monitoring and corrective action records within 7 working days after the records are created or within a reasonable timeframe, provided that the preventive controls qualified individual prepares (or oversees the preparation of) a written justification for a timeframe that exceeds 7 working days; and
(ii) Records of calibration, testing (e.g., product testing, environmental monitoring), supplier and supply-chain verification activities, and other verification activities within a reasonable time after the records are created; and
(5) Other activities appropriate for verification of implementation and effectiveness.
(b) Written procedures.
Specific procedures are required where product testing for a pathogen (or indicator organism) or other hazard is used as a verification activity
Specific procedures are required where environmental monitoring for a pathogen (or indicator organism) is used as a verification activity
- 117.180 Requirements applicable to a preventive controls qualified individual and a qualified auditor
The requirements for a PCQI are generally not covered in the BRCGS standard and need to be addressed
- a) One or more preventive controls qualified individuals must do or oversee the following:
(1) Preparation of the food safety plan (§117.126(a)(2));
(2) Validation of the preventive controls (§117.160(b)(1));
(3) Written justification for validation to be performed in a timeframe that exceeds the first 90 calendar days of production of the applicable food;
(4) Determination that validation is not required (§117.160(c)(5));
(5) Review of records (§117.165(a)(4));
(6) Written justification for review of records of monitoring and corrective actions within a timeframe that exceeds 7 working days;
(7) Reanalysis of the food safety plan (§117.170(d)); and
(8) Determination that reanalysis can be completed, and additional preventive controls validated, as appropriate to the nature of the preventive control and its role in the facility’s food safety system, in a timeframe that exceeds the first 90 calendar days of production of the applicable food.
Subpart F Requirements Applying to Records that must be Established and Maintained
- 117.305 General requirements applying to records.
The are some requirements for records that are not covered in the BRCGS standard and need to be addressed.
Records must identify:
- the date and time of the activity being documented
- signature/initials of individual performing the activity or conducting the record review
- information to identify the facility (e.g., name and location)
- the identity of the product and lot code where applicable.
- 117.310 Additional requirements applying to the food safety plan.
The owner, operator, or agent in charge of the facility must sign and date the food safety plan:
- 117.315 Requirements for record retention.
(a)
(1) All records required by this part must be retained at the plant or facility for at least 2 years after the date they were prepared.
Subpart G—Supply-Chain Program
- 117.405 Requirement to establish and implement a supply-chain program.
(a)
(1) Except as provided by paragraphs (a)(2) and (3) of this section, the receiving facility must establish and implement a risk-based supply-chain program for those raw materials and other ingredients for which the receiving facility has identified a hazard requiring a supply-chain-applied control.
Specific supplier approval and verification activities are required no matter how many times a required control is applied prior to being received at a site, the receiving site is responsible for verifying that the control has been applied.
- 117.140 Preventive control management components.
(b) The supply-chain program established in subpart G of this part is subject to the following preventive control management components as appropriate to ensure the effectiveness of the supply-chain program, taking into account the nature of the hazard controlled before receipt of the raw material or other ingredient:
(1) Corrective actions and corrections in accordance with §117.150, taking into account the nature of any supplier non-conformance;
(2) Review of records in accordance with §117.165(a)(4); and
(3) Reanalysis in accordance with §117.170.
- 117.415 Responsibilities of the receiving facility.
The site must identify and document of supplier verification activities that are not accepted in procedures.
- 117.420 Using approved suppliers.
(a) Approval of suppliers.
(b) Written procedures for receiving raw materials and other ingredients.
(1) Written procedures for receiving raw materials and other ingredients must be established and followed;
(2) The written procedures for receiving raw materials and other ingredients must ensure that raw materials and other ingredients are received only from approved suppliers (or, when necessary and appropriate, on a temporary basis from unapproved suppliers whose raw materials or other ingredients are subjected to adequate verification activities before acceptance for use); and
Verification activities must be conducted before receiving and using raw materials and ingredients on a temporary basis from unapproved suppliers.
- 117.430 Conducting supplier verification activities for raw materials and other ingredients.
When a hazard in a raw material or other ingredient is controlled by the supplier and is one for which there is a reasonable probability that exposure to the hazard will result in serious adverse health consequences or death to humans, an onsite audit of the supplier is required before using the raw material and at least annually thereafter.
(f) There must not be any financial conflicts of interests that influence the results of the verification activities listed in §117.410(b) and payment must not be related to the results of the activity.
(a) An onsite audit of a supplier must be performed by a qualified auditor.
- 117.475 Records documenting the supply-chain program.
(a) The records documenting the supply-chain program are subject to the requirements of subpart F of this part.
(b) The receiving facility must review the records listed in paragraph (c) of this section in accordance with §117.165(a)(4).
Pet Food Manufactures
Sites identified as BRCGS Category 11 for pet food need to comply with all requirements of the Preventive Controls for Human Food in Module 13 and the additional requirements of the Preventive Controls for Animal Food identified.
Food Defense: 21 CFR PART 121
Sites required to register with FDA as a food facility need to comply with all requirements for Food Defense identified in Module 13.
Sanitary Transportation: 21 CFR Part 1 Subpart 0
Sites need to ensure sanitary transportation practices for food transported by motor or rail vehicle within the US comply with the requirements for Sanitary Transportation in Module 13.
Produce Safety: 21 CFR Part 112
Sites identified as BRCGS Category 5 that have business operations that are regulated by 21 CFR Part 112 (Produce Safety rule), need to comply with all requirements for Produce Safety in Module 13.