The SQF Food Safety Code: Top Critical Non-Conformances from Certification Audits in 2022
The SQF Food Safety Code: Top Critical Non-Conformances from Certification Audits in 2022
The following is an analysis of Critical Non-Conformances found at SQF sites during SQF Certification audits in 2022. It is useful to be wary of these critical NC’s and ensure that your site is adequately prepared in these areas as a critical non-conformance raised at an initial certification audit results in an automatic failure of the audit, and your site is required to re-apply for certification. As a word of warning, there were 24 Critical NCs in 2022 (double the number in 2021), you will see from the summary below that the main areas you need to be aware of are:
2.4.3 Food Safety Plan (Mandatory)
11.2.4 Pest Prevention
2.1.1.2 Senior site management – food safety culture
No. 1 Critical Non-Conformance 2022 – 3 Critical NC’s Clause 11.2.4.3 from Section Pest Prevention
This clause requires pest activity risks to be analyzed and recorded. Inspections for pest activity shall be conducted on a regular basis. You are likely to receive a critical non-conformance if there is a risk of contamination to food products, raw materials, or packaging.
No. 2 Critical Non-Conformance 2022 – 2 x 11.2.4.4 from Section Pest Prevention
Along the same lines as the No. 1 critical NC, this clause requires that food products, raw materials, or packaging that are found to be contaminated by pest activity shall be effectively disposed of, and the source of pest infestation investigated and resolved.
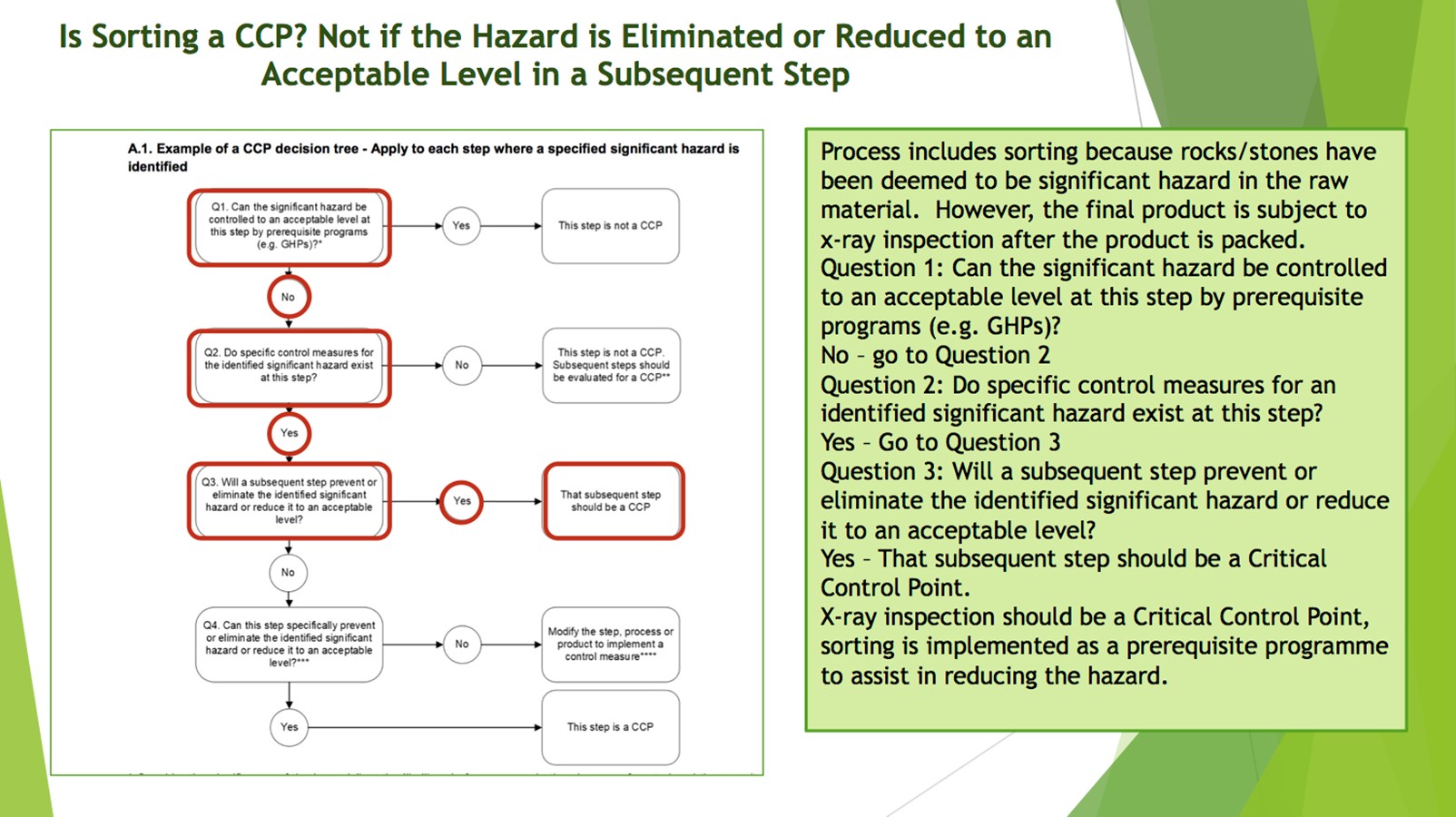
No. 3 Critical Non-Conformance 2022 – 2 x Clause 2.4.3.1 from Section Food Safety Plan (Mandatory)
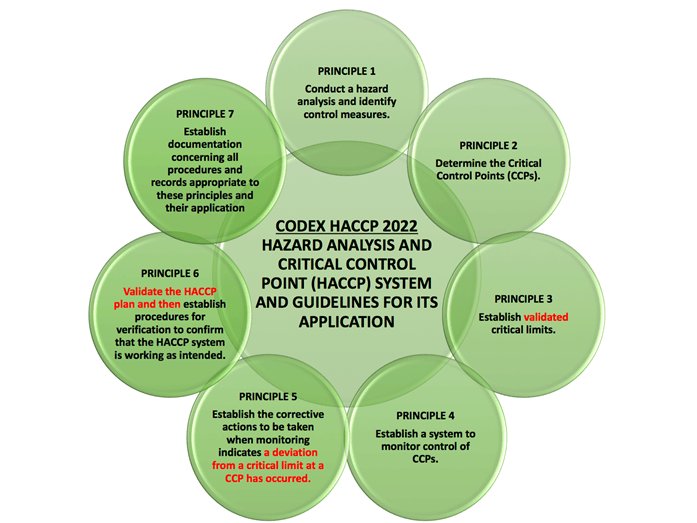
This clause requires a food safety plan to be prepared in accordance with the twelve steps identified in the Codex Alimentarius Commission HACCP guidelines. Clearly if you don’t have a food safety plan prepared according to CODEX guidelines then you probably should be certified although some alternative methods may be acceptable if justified.
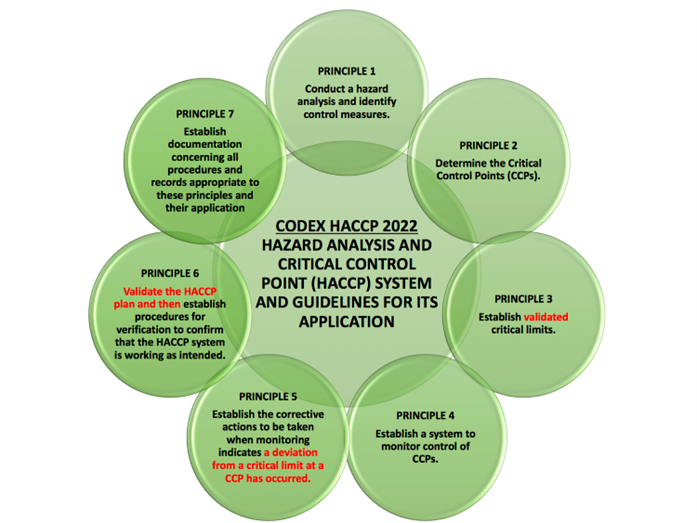
As a reminder, the 12 Steps Identified by CODEX are:
- Assemble HACCP Team and Identify Scope
- Describe product
- Identify intended use and users
- Construct flow diagram
- On-site confirmation of flow diagram
- List all potential hazards that are likely to occur and associated with each step, conduct a hazard analysis to identify the significant hazards, and consider any measures to control identified hazards (Principle 1)
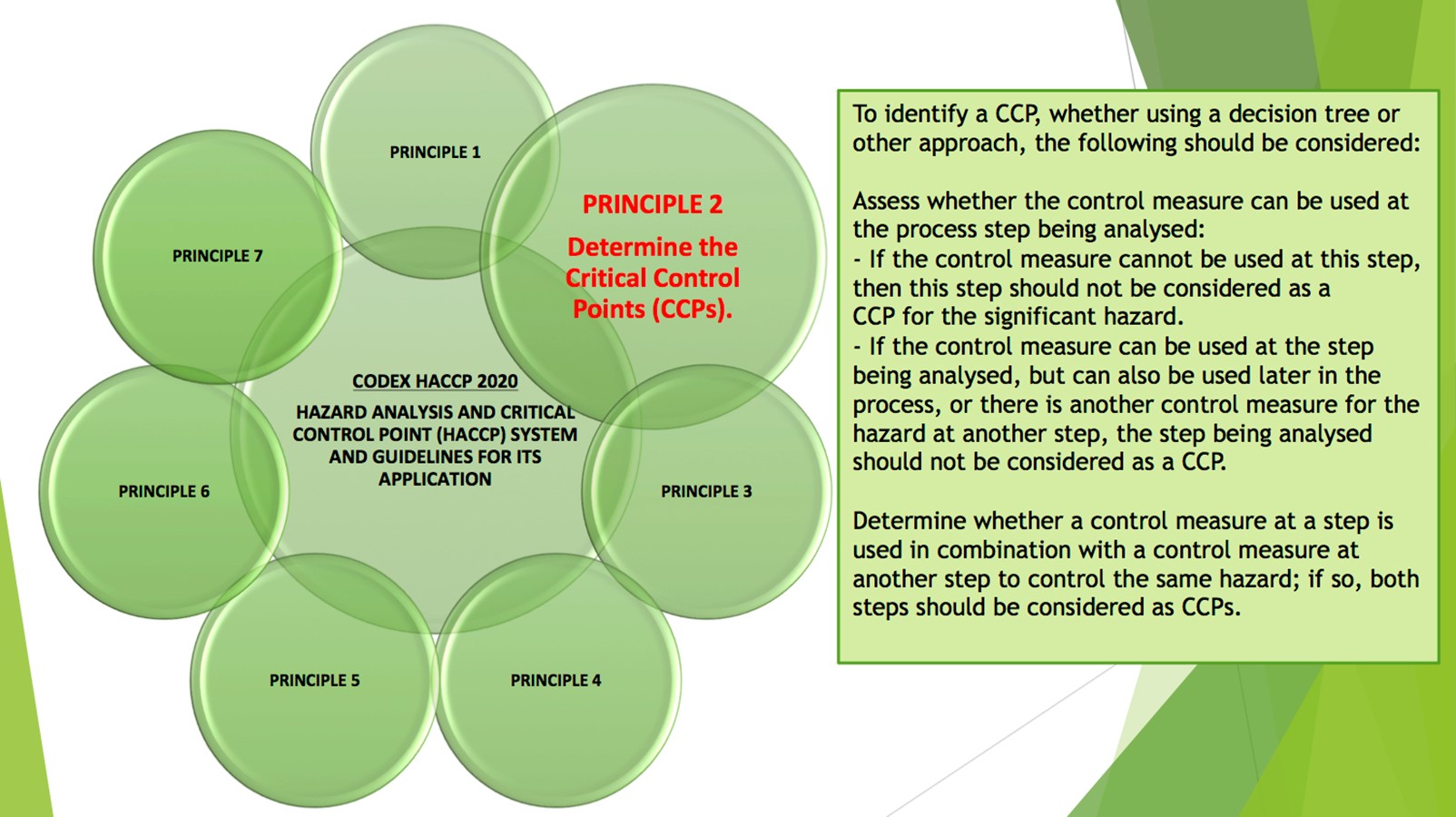
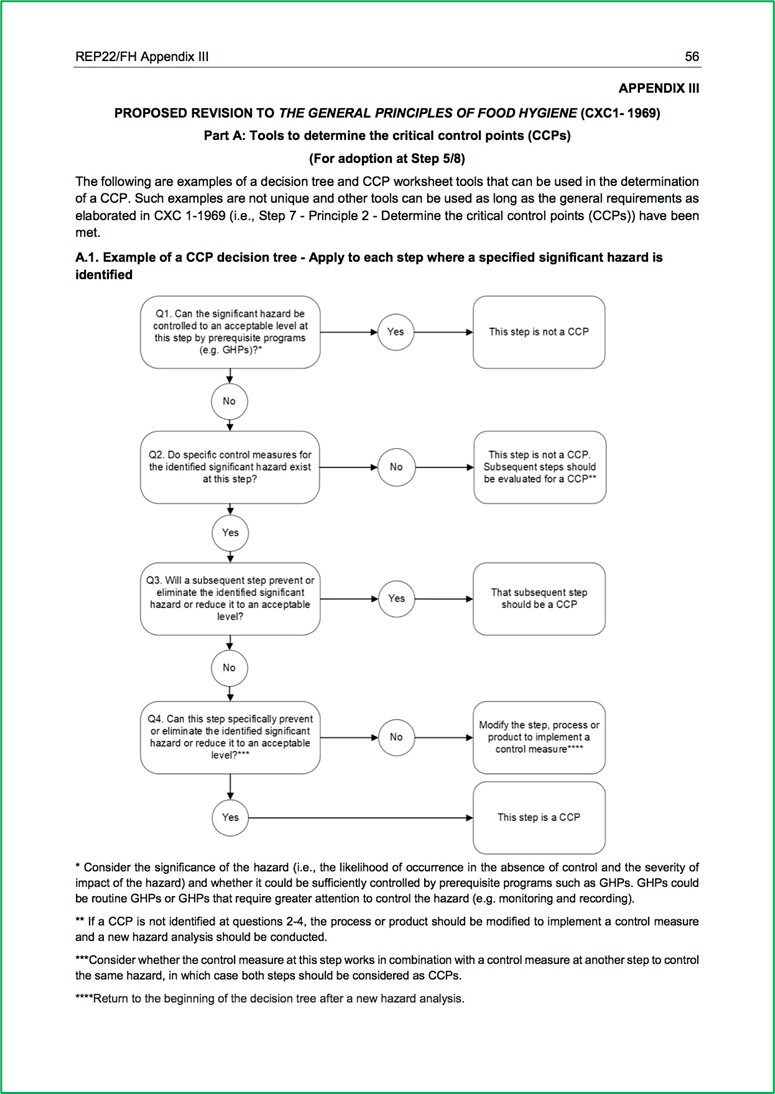
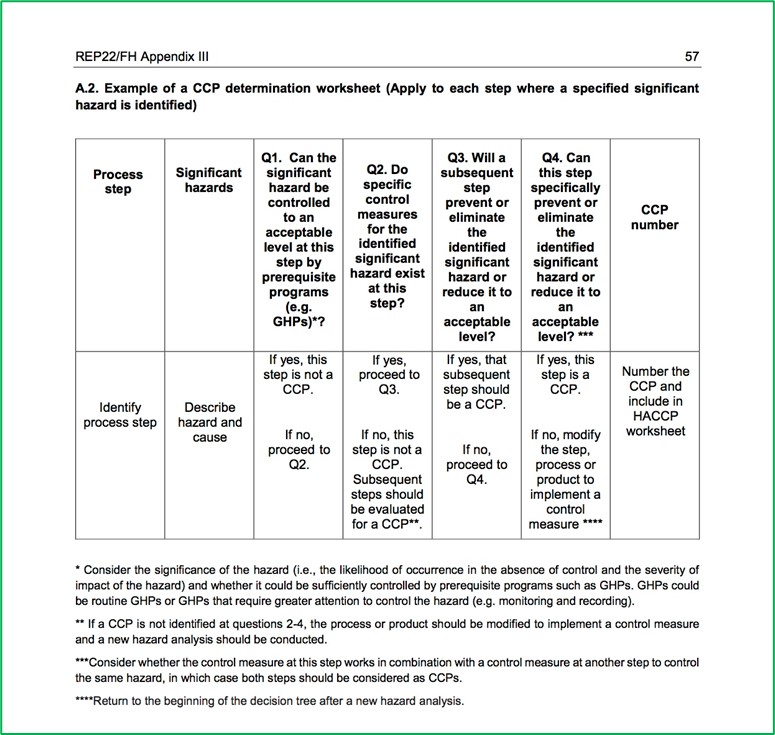
- Determine the Critical Control Points (Principle 2)
- Establish validated critical limits for each CCP (Principle 3)
- Establish a Monitoring System for Each CCP (Principle 4)
- Establish corrective actions (Principle 5)
- Validation of the HACCP Plan and Verification Procedures (Principle 6)
- Establish Documentation and Record Keeping (Principle 7)
No. 4 Critical Non-Conformance 2022 – 2 x Clause 2.4.3.10 from Section Food Safety Plan (Mandatory)
This clause requires the food safety team to identify the steps in the process where control must be applied to eliminate a significant hazard or reduce it to an acceptable level (i.e., a critical control point or CCP). Clearly a failure to identify a critical control point is serious and result in a Critical Non-Conformance.
No. 5 Critical Non-Conformance 2022 – Clause 11.2.4.1 from Section Pest Prevention
This clause requires a documented pest prevention program to be effectively implemented. That is three of the top five related to pest management.
No. 6 Critical Non-Conformance 2022 – Clause 2.1.1.2 from Section Management Responsibility (Mandatory)
This clause requires Senior Site Management to lead and support a food safety culture within the site. This is relatively new requirement for food safety management system certification schemes and obviously caught a few sites out in 2022.
No. 7 Critical Non-Conformance 2022 – Clause 2.4.3.11 from Section Food Safety Plan (Mandatory)
This clause is again related to food safety plans, for each identified CCP, the food safety team need to identify and document the limits that separate safe from unsafe product (critical limits).
No. 8 Critical Non-Conformance 2022 – Clause 2.4.3.12 from Section Food Safety Plan (Mandatory)
This clause is again related to food safety plans, the food safety team need to develop and document procedures to monitor CCPs to ensure they remain within the established limits.
No. 9 Critical Non-Conformance 2022 – Clause 2.4.3.14 from Section Food Safety Plan (Mandatory)
This clause is again related to food safety plans, the documented and approved food safety plan(s) shall be implemented in full.
Source of Information
Tammie Van Buren
Compliance Manager, SQFI Food Marketing Institute
Food Safety Trends and Challenges – Insights from SGS
https://www.youtube.com/watch?v=avTmwczfx80
SQF Food Safety Management System Implementation Packages
Our SQF packages contain comprehensive top level Food Safety Management procedures templates in Microsoft Word English (US) format that form the foundations of your Food Safety Management System so you don’t have to spend 1,000’s of hours writing compliant procedures. Over 70 top level documents that cover all the requirements of Modules 2 & 11 of the SQF Code Edition 9 and match the clauses of the code for ease of implementation.
The packages also include supplementary documentation to FS 2.4.3 Food Safety Plans (19 page HACCP procedural template) including the SQF HACCP Calculator and Instructions. The HACCP documentation included enables the user to develop food safety plans in accordance with the twelve steps identified in the Codex Alimentarius Commission HACCP guidelines as per the specific requirement of the new SQF Code Edition 9.