IFS Food Standard Version 7
International Featured Standards (IFS) are one of the major recognized food safety management certification schemes with over 26,000 IFS certificates in 90 different countries being issued per year.
First developed in 2003 the IFS Food Standard is a GFSI (Global Food Safety Initiative) recognized Standard for assessing product and process compliance in relation to food safety and quality. The standard is applicable to food manufacturers and companies that pack loose food products.
The aim of IFS Food Certification is to assess whether the processing activities of a manufacturer are able to produce products that are safe, legal and in compliance with customer specifications. IFS Food Certification is specific to site and all products and processes of the relevant are included in the scope of the IFS Food Assessment.
Certification to the IFS Food Standard requires an on-site evaluation, of which at least 50% of the audit duration is within the site production areas.
The evaluation includes inspection of external areas, goods receipt, production processes*, storage areas and dispatch areas.
There are checks to ensure Good Manufacturing Practices (GMP), including maintenance, hygiene, pest control and cleaning and disinfection activities are in place and that staff facilities are adequate and hygienic.
*Inspection of Operations: there is observation of running production lines and the control and monitoring of key process parameters including critical control points (CCPs).
During the evaluation, the auditor observes and interviews employees.
Following on-site evaluation there is a documentation and record review to verify the auditors finding during the inspection and assess if the documentation and record keeping also comply with the requirements of the IFS Food Standard.
The requirements of the IFS Food Standard are now documented in Version 7 which is effective for audits from 1st July 2021
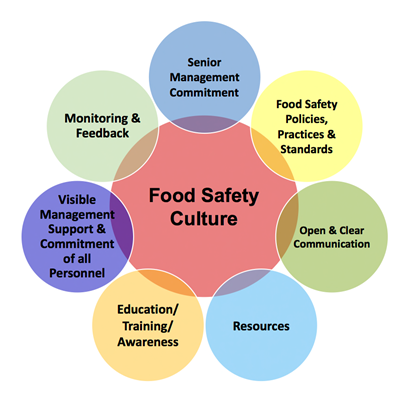
Version 7 is in alignment with GFSI Benchmarking Requirements Version 2020. The Global Food Safety Initiative (GFSI) Benchmarking Requirements are a widely-accepted benchmark for food safety certification programs. GFSI-recognized Certification Program Owners are required to address each key element outlined in GFSI Benchmarking Requirements Version 2020, the main change being the requirement for a food safety culture. Part III of the GFSI Benchmarking Requirements defines the key elements required in a Certification Program in relation to:
– Hazard and Risk Management Systems (Hazard Analysis and Critical Control Points (HACCP) or HACCP based systems)
– Food Safety Management Systems;
– Good Industry Practices, Good Manufacturing Practices, Good Agricultural Practices.
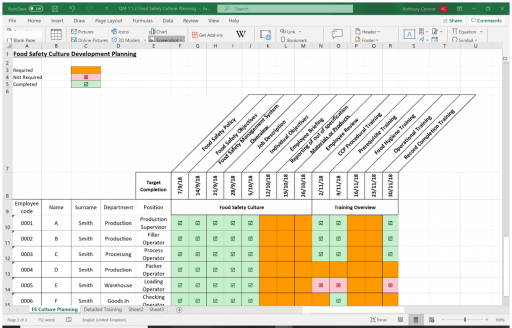
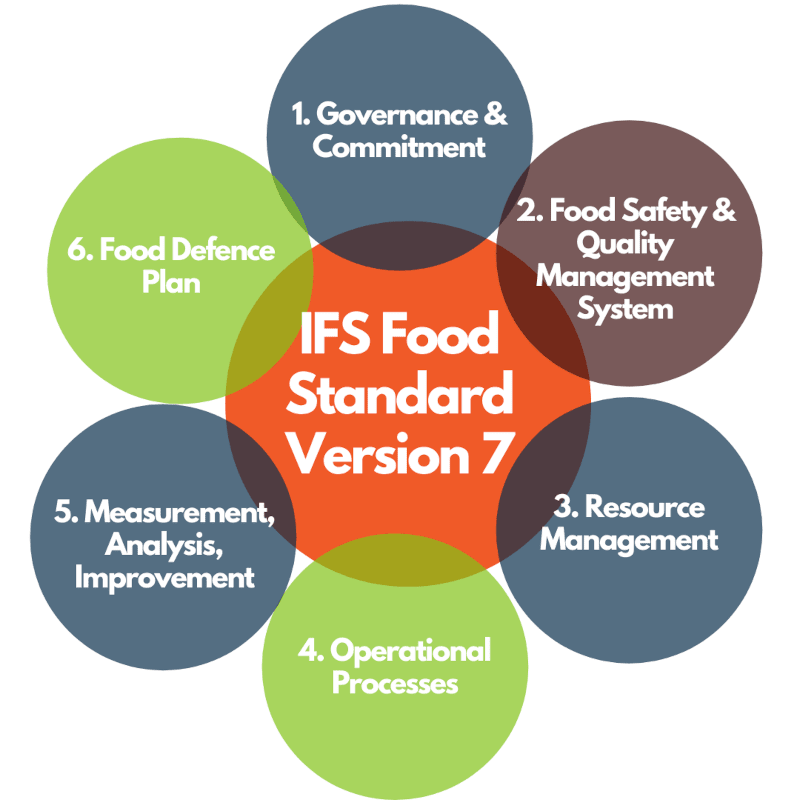
The requirement of the IFS Food Standard are laid out in 6 sections of the standard:
- Governance and Commitment
- Food Safety and Quality Management System
- Resource Management
- Operational Processes
- Measurements, Analyses, Improvements
- Food Defence Plan
Governance and Commitment
Section 1 Governance and Commitment has requirements for a Corporate Policy which should be declaring company commitment to its’ customers, food safety, product quality and to develop a food safety culture.
There needs to be a defined Corporate Structure including an organisational chart, employees need to aware of their responsibilities and these needs to be documented.
The site needs to retain Customer Focus, identify fundamental needs and expectations of customers and this information used for continuous improvement.
The senior management need to conduct Management Reviews of the food safety and quality management system at least annually, or when significant changes occur.
Food Safety and Quality Management System
The Food Safety and Quality Management System needs be documented and implemented
Documents need to be legible, unambiguous and comprehensive and controlled
Records and documented information need to be legible, genuine and securely retained for appropriate periods.
The food safety management system must be supported by HACCP Plan(s), based on Codex Alimentarius HACCP principles and any legal requirements
Resource Management
Sufficient Human Resources need to be provided. All personnel performing work that affects product safety, quality and legality must be competence and have documented responsibilities and job descriptions.
Documented requirements relating to Personal Hygiene need to be in place and applied. The requirements should be appropriate to the products/processes.
There needs to be documented Training and/or Instruction programs that apply to all personnel.
Adequate hygienic Staff Facilities need to be provided in sufficient quantity for the number of personnel.
Operational Processes
Requirements related to food safety and product quality, agreed with customers must be defined in a Contract Agreement.
There must be Specifications for raw materials, rework and finished products.
Formulas/Recipes agreed with customers must be complied with.
Procedures need to be in place to control Product Development/Product Modification/Modification of Production Processes including a hazard analysis and assessment of associated risks.
Procedures need to be in place to control Purchasing of raw materials, semi-finished products, packaging materials, outsourced processes and services which have an impact on food safety and product quality.
Key parameters for the Packaging materials based on hazard analysis, assessment of associated risks and intended use must be defined in specifications
There must be checks to verify the suitability and legality of packaging including relevant hazards or risks. Evidence needs to be available to demonstrate that packaging materials are suitable for use.
There are specific requirements for the Factory Exterior and Location, the Plant Layout and Process Flow including a Site Map and Production and Storage Premises (including constructional requirements, walls, floors, ceilings, overheads, windows, doors, lighting, ventilation, water, air and gases)
Based on hazard analysis and assessment of associated risks, documented Cleaning and Disinfection schedules must be implemented and monitored.
A Waste Management controls and procedures need to be in place to avoid cross contamination and comply with legal requirements.
Products must be protected against physical contamination. Foreign Material Risk Mitigation procedures and systems based on hazard analysis and assessment of associated risks must be in place to avoid contamination with foreign materials.
Foreign body detection equipment needs to be managed to ensure accuracy and efficiency, any potentially contaminated products need to be quarantined as non-conforming products pending further investigation.
Glass and Brittle Materials need to be excluded, if the presence of glass and/or brittle materials cannot be avoided, the risks need to be controlled.
Based on the risks, control measures need to be in place for the Handling of Glass Packaging, such measures include inversion, blowing, washing or rinsing.
Site infrastructure and operations must be designed and built to prevent pest infestation and Pest Control measures in compliance with legal requirements should be in place to minimise the risk of pest infestation.
All incoming goods, including packaging materials and labels, need to be subject to documented checks on Receipt.
The Storage of raw materials, semi-finished, finished products and packaging materials needs to be as per product specifications and not have any negative impact on other products. FIFO/FEFO stock rotation policies need to be in place.
Vehicles need to be in good condition, hygienic and subject to documented checks before loading, unloading and during transport. Procedures to prevent contamination should be in place and vehicles need to maintain the product temperatures during transport (if applicable). Product should be secure at all times.
Maintenance plans must be in place including critical equipment and vehicles. Repairs need to be controlled to prevent product contamination.
Equipment must be designed and specified for the intended use and verified during commissioning.
A Traceability System must in place that enables the identification of products and the ability to trace back through all stages of the process to batches of raw materials and primary packaging materials. The system must be tested, at a minimum, annually.
Based on risk assessment preventive control measures must be in place from receipt to dispatch, to ensure that potential cross contamination of products by Allergens is minimised.
A documented food fraud vulnerability assessment needs to be conducted and a subsequent documented Food Fraud Mitigation Plan implemented
Measurements, Analyses, Improvements
An Internal Audit Program with a scope and frequency based on risk must be in place to cover at least all the requirements of the IFS Standard.
Site and Factory Inspections must be carried out with a scope and frequency based on risk
Process and Working Environment Parameters that ensure the food safety and product quality need to be monitored and recorded.
Any measuring and monitoring devices required to ensure compliance with food safety and product quality requirements must be controlled, checked and Calibrated.
Quantity Control measures need to ensure that products to meet legal requirements of the destination country and customer specifications.
Risk based Testing Plans must be in place to ensure that product safety, quality, legal and specific customer requirements are met.
Laboratories with appropriate accredited programs/methods (ISO/IEC 17025) should be conducting analyses which are relevant for food safety, if not the results must be verified on a regular basis by laboratories accredited to ISO/IEC 17025 or equivalent.
Results of analyses must be evaluated promptly by competent personnel and corrective actions taken when results are unsatisfactory.
Quarantine procedures need to ensure that only raw materials, semi-finished and finished products, and packaging materials conforming to product requirements, are processed and dispatched.
Procedures need to be in place to handle Customer Complaints and notifications from authorities. Complaints and complaint trends should be analysed to prevent a recurrence and instigate actions for improvements
Procedures to deal with Incidents and Potential Emergency Situations with an impact on food safety, quality and legality need to be in place. Effective systems for the withdrawal and/or the recall of all products need to be available.
Systems need to be in place to Control Non-Conformities and Non-Conforming Products.
Documented Corrective Actions need to be undertaken to avoid the further occurrence of non-conformities.
Food Defence Plan
A Food Defence Plan needs to be developed and implemented based on a risk assessment of potential threats. The effectiveness of the plan needs to be monitored by internal audit and inspection
Procedures for managing External Inspections and Regulatory Visits should be in place.