
How to implement a HACCP System
Introduction
A HACCP System ensures that all food safety hazards, that may reasonably be expected to occur, are identified by this process and are then fully evaluated and significant hazards that have been identified are controlled so that products do not represent a direct or indirect risk to the consumer. Resultant control measures are implemented through the HACCP (food safety) plan and where applicable Prerequisites/GMPs. Below is the process of establishing an effective HACCP System.
Prerequisite Programs/GMPs
Environmental and operational programs necessary to create an environment suitable to produce safe and legal food products should be established. Good Manufacturing Practices that should be implemented prior to Hazard Analysis include:
- Environment controls
- Construction and layout of buildings and utilities
- Layout of premises, including workspace and employee facilities
- Supplies of air, water, energy and other utilities
- Supporting services, including waste and sewage disposal
- Suitability of equipment
- Management of purchased materials
- Measures for the prevention of contamination/cross-contamination
- Cleaning and sanitizing
- Pest control
- Personnel hygiene
- Control of rework
- Product recall procedures
- Warehousing
- Product information and consumer awareness
- Food defense, bio vigilance and bioterrorism
- Training and Supervision
HACCP Preliminary Steps
1. Assemble the HACCP team, with at least one team member who is HACCP trained A core multidisciplinary team should be utilized within the company to develop the Food Safety Management System. This core team should be supplemented by other staff when specific areas or products are being analyzed. 2. Make a description of the product, how it is processed or manufactured and the storage and distribution process The HACCP team should document the end product characteristics, including legal food safety requirements, for the purpose of conducting the Hazard Analysis. 3. Identify the intended use of the products The HACCP team should identify all possible users and consumers for each product and process category. Vulnerable groups of the population may have to be considered. The HACCP team should consider the consumers of the product:
- Is the product intended for babies or infants, children or adults?
- Is the product intended for a wide spectrum of the population?
- Is the product likely to be consumed by high risk groups?
4. Identify consumers of the products The intended use should be based on the expected uses of the product by the end user or consumer. Consider the intended use of the product:
- Is the product intended as an ingredient for further cooking?
- Is the product ready to eat?
5. Confirm the HACCP Scope HACCP team should define the scope of the HACCP study. For each different type of product or process the HACCP team should define the scope of each HACCP plan, including the products and processes covered. 6. Consider the process and draw a flow diagram The HACCP Team should construct flow charts for the products and process categories covered by the scope of the food safety management system. For each step in the flow chart the Food Safety team should describe the step and the control measures. 7.Confirm the flow diagram is correct by following the process Flow charts should be physically confirmed by the HACCP (Food Safety) Team
HACCP principles
All processes used in the manufacture of food products and product groups should be subject to hazard analysis incorporating the Codex Alimentarius HACCP principles
Principle 1
Prepare a flow diagram of the steps in the process. Conduct a hazard analysis by identifying potential hazards. Assess likelihood of occurrence of these hazards and identify control options.
Principle 2
Identify the Critical Control Points in the process using the decision tree.
Principle 3
PEstablish critical limits, which must be met to ensure each Critical Control Point is under control .
Principle 4
Establish a monitoring system to ensure control of the Critical Control Point by scheduled testing or observations.
Principle 5
Establish the corrective action to be taken when monitoring indicates that a particular Critical Control Point is moving out of control.
Principle 6*
Establish documentation concerning all procedures and records appropriate to these principles and their application.
Principle 7*
Verify that HACCP is working effectively. * Note that in CODEX Annex Guidelines Principle 6 and Principle 7 are verify then document, from a practical point of view I find it easier to document then establish verification procedures which should also be documented. The following steps should be implemented in establishing the HACCP System:
Hazard Analysis
The HACCP team should conduct a hazard analysis for food safety hazards that are reasonably likely to occur for each product and process category. Taking the confirmed process flow diagram your HACCP team will now need to conduct a Hazard Analysis for each step to identify the threats to human health, which might be introduced into products as they are produced. Hazards are predominantly grouped into three categories:
- Biological (including microbiological)
- Chemical
- Physical
Allergens and radiological hazards may also need to be considered. The next step in performing a hazard analysis is for the HACCP team to consider the list all of the hazards that may be reasonably expected to occur at each step. This first step in identifying hazards which might be associated with your production process might be considered a “brainstorming” session. For each Food Safety Hazard Identified, the acceptable level of the hazard in the end product is determined taking into account:
- Regulatory requirements
- Customer food safety requirements
- Historic information
- Scientific literature
- Professional experience
- Intended use by the customer
This hazard list is referred to as a Preliminary Hazard List and covers all hazards that could potentially occur in the product. In conducting the hazard analysis, wherever possible the following should be considered:
- The probability of hazards occurring
- The severity of hazards by their adverse health effects
- The qualitative/quantitative evaluation of the presence of hazards
- Survival or multiplication of microorganisms of concern
- Production or persistence of toxins, chemicals or physical agents
- Conditions leading to the above
- Customer complaints and previous internal non-conformances
- Prerequisite programs that create hygienic and safe conditions
The HACCP team must then consider what control measures, if any, exist which can be applied for each hazard. Each potential food safety hazard should now be risk assessed by the Food Safety Team to determine whether its elimination or reduction to acceptable levels is required to produce a safe product and also any controls required to achieve the acceptable levels.
Hazard Assessment
Each potential food safety hazard is risk assessed to determine whether its elimination or reduction to acceptable levels is required to produce a safe product and also any controls required to achieve the acceptable levels. For each step grades of impact (severity of adverse health effects) and probability (likelihood of a food safety hazard occurring) need to be allotted and the combined matrix used to judge the significance and priority for elimination or minimization of the hazard. First the Food Safety Team assess the probability of the hazard occurring and enter: 1 for Highly Unlikely 2 for Possible 3 for Likely Then the Food Safety Team assesses the severity of the hazard and enters: 1 for Not Severe 2 for Could possibly cause illness 3 for Severe (Could be fatal) Probability and Severity are Multiplied to give a Significance Score for the Hazard. All of the food safety hazards that score a 9 are regarded as significant and form the Significant Food Safety Hazard List
Critical Control Points
HACCP Principle 2 is to identify the critical control points in the process. A CCP is a step in a food process at which control can be applied to prevent, eliminate, or reduce to acceptable levels a food safety hazard. Critical Control Points are established using the decision tree as the latest step in the flow path where controls can be effectively administered for a particular Significant Food Safety Hazards.
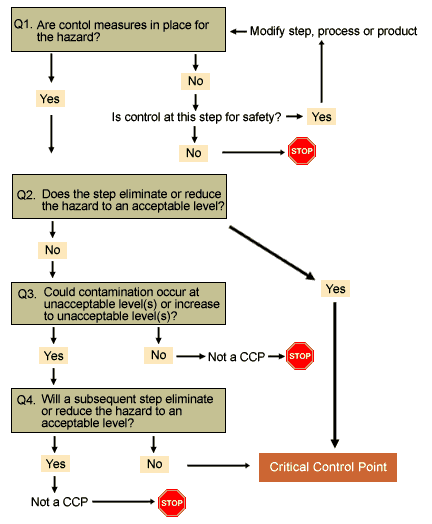
Note: You should still ensure that your control measure or prerequisite programs adequately control significant hazards even if not identified as controlled at CCPs. Each hazard on the Significant Food Safety Hazard list must be controlled by a control measure (or combination of control measures) that prevent, eliminate or reduce the hazard to the defined acceptable levels.
Critical Limits for each CCP
For each CCP, the appropriate critical limits are defined. A critical limit is the maximum or minimum value to which a physical, biological, or chemical hazard must be controlled at a critical control point to prevent, eliminate, or reduce to an acceptable level the occurrence of the identified food safety hazard. Critical limits are exact and specify the limits required for food safety using the preventive measures put in place at CCPs. A critical limit can be an upper limit where a set amount or level cannot be exceeded. A critical limit can also be a lower limit where a minimum amount is required to produce the safe effect.
Validation of Control Measures
The HACCP team should confirm that the control measures (or combination of control measures) are capable of achieving the defined acceptable levels for each food safety hazard by validation activities. Supporting validation documentation can consist of information from:
- Regulatory limits or Industry Code of Practice Guidelines
- Scientific journals
- Documented challenge studies
- In-house data
The HACCP documentation must identify:
- The hazard or pathogen, including the level of hazard prevention or pathogen reduction to be achieved
- The processing steps that will achieve the specified reduction or prevention
Establishing a Monitoring System for each CCP
A monitoring procedure should be established for each CCP to ensure compliance with critical limits. The most commonly recognised monitoring procedures are from instruments but can be employee checks such as inspecting the documentation accompanying incoming materials. Continuous monitoring is always preferred when it is available. This is normal when the process is continuous rather than by batch. Monitoring should ideally provide information in time to make adjustments to ensure control of the process to prevent it exceeding the critical limits. Ideally adjustments should be taken before a critical limit is breached.
Establishing a Corrective Action Plan
The corrective action to be taken when monitored results indicate a failure to meet a control limit is defined including responsibilities. The corrective action plan needs ensure:
- the cause of the deviation has been identified and eliminated
- the CCP reverts to a controlled state after the corrective action has been taken
- measures to prevent recurrence of the deviation have been established
- product is quarantined until it is established that it is safe
Establishing HACCP Documents and Records
The HACCP team should establish procedures and records to ensure adequate food safety controls are in place. This includes documenting the HACCP plan which summarises all the critical control points, the monitoring procedures, critical limits, corrective actions, records and responsibility and authority.
Verification Planning
The HACCP team should define the methods, frequencies and responsibilities for verification activities (the simplest way to do this is by review of product analysis results and/or audit of HACCP documentation).
Review of the HACCP Plan
The HACCP team should review the HACCP plan and prerequisite programs at least annually and prior to any changes which may affect food safety.
References
“Hazard Analysis and Critical Control Point (HACCP) system and Guidelines for its Application” (Codex Alimentarius Commission, Geneva).


