
How to Develop a Food Safety Culture
Food Safety Culture
A successful food safety culture is the product of individual and group values, attitudes, competencies and patterns of behaviour that determine the commitment to, and the style and proficiency of the food safety management system. Senior management should plan for the development and continuing improvement of a food safety culture.
Senior management should be implementing a “It is how we do things here” food safety culture. This can be achieved by:
- Leadership – starting from the top
- Demonstrating visible commitment
- Effective communication of company philosophy and policy
- Ensuring there is accountability from the top of the organization to the bottom
- Developing employee confidence and mutual trust
- Developing reward schemes including ‘Employee of the Month’ award
- Ensuring all employees are accountable, engaged and understand the value of integrity and proactivity
- Developing an action plan for the development and continuing improvement of food safety culture
To ensure success Senior Management should be directly responsible for food safety by ensuring adequate; organization and support, equipment and facilities, training and education of all employees, reviewing and auditing performance, and driving continuous improvement.
All employees should be empowered and individually responsible for the quality of their work, resulting in a continual improvement culture and working environment for all. Employees should be encouraged and required to notify management about actual or potential food safety issues and are empowered to act to resolve food safety issues within their scope of work.
The philosophy of Food Safety should be promoted throughout the organization and in particular the Food Safety Policy.
Communication processes for promoting food safety include:
- Team briefings
- Staff reviews
- Daily Management meetings
- Feedback mechanisms
- Newsletters
- Notice boards
Senior management should monitor and measure through reports and trend analysis the degree of development of the food safety culture by analyzing information including KPIs from:
- Hygiene & Housekeeping Audits
- Internal Audits
- External Audits
- Non-conforming products
- Environmental monitoring
- Review of implementation plan and numbers trained
- Employee reviews
- Staff surveys on values and culture
- Customer Complaints
- Staff Turnover
- Staff Exit Interviews
All employees should undergo individual food safety culture development which can include:
- Food Safety Policy
- Food Safety Objectives
- Food Safety Management System Overview
- Job Descriptions
- Job Training
- Employee Briefing
- Individual Objectives
- CCP Controls – Training Procedures & Record Completion
- PRP Controls – Training Procedures & Record Completion
- Employee Review
A training matrix can be used for Food Safety Culture Planning:
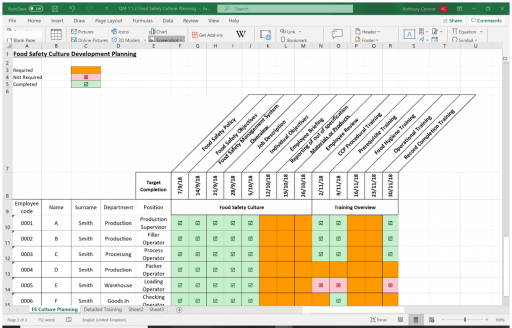
Records of all training should be maintained, including those of induction, on-the-job, refresher and external training. Training schedules and records should be managed by Department Managers and where applicable include the following records:
- Training register
- Operator training review
- Training matrix
- Department training matrix
- Individual Training records including:
- Description of training
- Skills description
- Name of trainee
- Confirmation of training
- Date and duration of training
- Trainer details
- Verification that the trainer has assessed the trainee and found them to be competent
- Identifying the competencies needed for specific roles
- Reviewing and auditing the implementation and effectiveness of the training and the competency of the trainer with a view to taking action to improve the training.












