SQF Edition 9 Checklist: Every Document You Need for a Perfect Audit
Having helped approaching 1,000 organizations achieve SQF (Safe Quality Food) Certification, we know that it is a monumental milestone for any food business. However, the transition to Edition 9 brought significant changes, specifically regarding Food Safety Culture, Product Development, Product Release and Practitioner accountability.
To achieve an “Excellent” rating (96–100), your documentation must do more than just exist—it must be appropriate, operational, verifiable, and validated. Use this comprehensive checklist to ensure your site is ready for the SQF auditor.
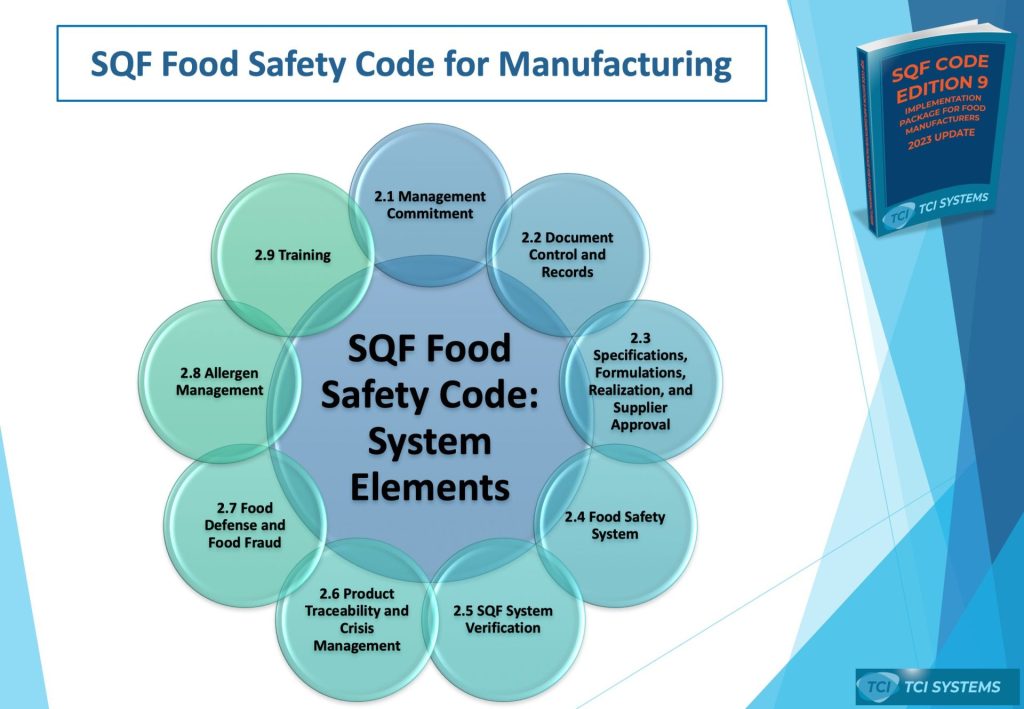
Part 1: Module 2 – System Elements (The "Brain")
Module 2 is the management core of your SQF food safety management system. These documents prove that your leadership is committed and your food safety system is structured to meet the requirements of the SQF Food Safety Code.
SQF Edition 9 Checklist Part 1: Module 2 – System Elements
- Management Commitment & Culture
- [ ] Food Safety Policy Statement: Signed by the senior site manager and displayed prominently. It must include a commitment to supply safe food, comply with customer and legislation requirements, and establish a food safety culture.
- [ ] Organizational Chart: Clearly defining the reporting structure and identifying backups for all key food safety roles.
- [ ] Job Descriptions: Documented for all personnel with food safety responsibilities.
- [ ] Food Safety Culture Plan: Documentation showing how the site measures and improves its culture (e.g., surveys, incentive programs).
- [ ] Food Safety Objectives: Agreed by the senior site management and cascade throughout the organization. Objectives need to be SMART: Specific, Measurable, Achievable, Relevant, and Time-bound.
- The SQF Practitioner Requirements
- [ ] Practitioner Designation: Evidence that a primary and substitute practitioner are employed full-time.
- [ ] Practitioner Qualification/Knowledge: Evidence that the primary and substitute practitioner have knowledge and/or experience of the SQF Food Safety Code.
- [ ] HACCP Training Certificates: Verifiable proof of formal HACCP training for both practitioners.
- [ ] Monthly Management Updates: Records of the practitioner updating senior management on the SQF system’s performance.
- Document Control & Records
- [ ] Document Register: A master list of all current SOPs, forms, and policies.
- [ ] Record Retention Policy: Documented periods for record-keeping (minimum of the product’s shelf-life).
- Specifications & Supplier Approval
- [ ] Raw Material, Packaging & Finished Product Specs: Current, approved specifications for every item entering the facility.
- [ ] Approved Supplier Register: A list of validated suppliers and the risk-based methods used to approve them.
- [ ] Contract Manufacturer Agreements: (If applicable) Signed agreements and audit records for third-party processors.
- Food Safety Plan (HACCP)
- [ ] Product Descriptions: Including intended use and vulnerable populations.
- [ ] Flow Diagrams: Verified by the HACCP team within the last 12 months.
- [ ] Hazard Analysis: Based on CODEX HACCP Principles and Identifying biological, chemical, and physical risks.
- [ ] CCP Monitoring Records: Daily logs showing critical limits are met.
- Complaint Management (Mandatory)
- [ ] Documented Procedure, Records of Complaints, Complaint Investigation, Corrective Action Records and Trend Analysis.
- Product Sampling, Inspection, and Analysis
- [ ] Documented Procedures, Recognized Standard Methods, Sampling Plans, Records of Analysis/Inspection and Proficiency Testing.
- Non-Conforming Materials and Product
- [ ] Documented Procedure including Quarantine of Suspect Materials/Product and Records of Disposal or Alternate Use.
- Product Rework
- [ ] Documented Procedure and Records including Identification and Trace of Materials/Product.
- Product Release (Mandatory)
- [ ] Documented Procedure and Records including Release by Authorized Personnel.
- Corrective and Preventative Action (Mandatory)
- [ ] Documented Procedure and Records including Root Cause Analysis for the resolution of non-compliances/potential non-compliances.
- Internal Audits and Inspections (Mandatory)
- [ ] Documented Schedule and Procedure covering all aspects of the SQF Food Safety Code.
- [ ] Internal Audit Reports: Showing a full audit of the SQF system conducted annually.
- [ ] Documented Schedule, Procedure and Records for Inspections of Site, Fabric and Hygiene to verify Good Manufacturing Practices are compliant, and the facility & equipment maintained in a satisfactory condition.
- Product Identification (Mandatory)
- [ ] Documented Procedure and Records to identify raw materials, ingredients, packaging, work-in-progress, process inputs, and finished products during all stages of the operation.
- [ ] Documented Product Start-Up, Product Changeover, and Packaging Changeover Procedure and Records.
- Product Trace (Mandatory)
- [ ] Documented Procedure and Records to Track all Stages from Material Receipt to Delivery to Customer and vice versa.
- Product Withdrawal and Recall (Mandatory)
- [ ] Documented Procedure and Records for Initiating, Managing, and Investigating a Product Withdrawal or Recall.
- [ ] Annual Mock Recall: Documentation of a trace-back and trace-forward (target: 4 hours or less).
- Crisis Management Planning
- [ ] Documented Procedure and Records for dealing with Events that could impact on the site’s ability to deliver safe food such as flood, drought, fire, computer outage, or loss of electricity or refrigeration.
- Food Defense Plan (Mandatory)
- [ ] Documented Food Defense Threat Assessment.
- [ ] Documented Food Defense Plan, and corresponding Procedures and Records.
- Food Fraud (Mandatory)
- [ ] Documented Food Fraud Vulnerability Assessment.
- [ ] Documented Food Fraud Mitigation Plan, and corresponding Procedures and Records.
- Allergen Management (Mandatory)
- [ ] Documented Risk Analysis of Raw Materials
- [ ] Documented Assessment of Workplace-Related Food Allergens
- [ ] Documented List of Allergens on Site
- [ ] Documented Allergen Control Procedures and Records
- [ ] Documented Validation and Verification of Cleaning & Sanitation Procedures and Records when required to prevent Cross-Contact.
- [ ] Documented Finished Product Label Checks to ensure that labels are correct with regard to allergens.
- Training Program (Mandatory)
- [ ] Documented Training Program and Records which include competencies for specific duties and the training methods for tasks critical for ensuring compliance with the SQF Food Safety Code.

Part 2: Module 11 – Good Manufacturing Practices (The "Body")
Module 11 focuses on the physical environment and operational habits. The auditor will look for these records during the facility walkthrough.
SQF Edition 9 Checklist Part 2: Module 11 – Good Manufacturing Practices Checklist
- Personnel & Hygiene
- [ ] Personnel Hygiene Policy: Covering jewelry, handwashing, and smock procedures.
- [ ] Visitor/Contractor Records: Documented sign-ins and hygiene briefing for all guests.
- [ ] Training Matrix: A master record showing who was trained, when, and by whom for every GMP.
- Facility & Equipment Maintenance
- [ ] Preventive Maintenance (PM) Schedule: A calendar of planned maintenance for all food-contact equipment.
- [ ] Calibration Log: Records for thermometers, scales, and metal detectors.
- [ ] Glass & Brittle Plastic Register: Monthly inspection records of all overhead lights and windows.
- [ ] Documented Procedure and Records for managing Utilities including Water and Compressed Air
- Pest Prevention
- [ ] Documented Pest Prevention Program, Contract, Methods, Records and Site Map of Pest Prevention & Monitoring Devices
- [ ] Pest Control Service Reports: Including trend analysis
- Cleaning & Sanitation
- [ ] Master Sanitation Schedule (MSS): Detailing what is cleaned, how often, and who is responsible.
- [ ] SSOPs (Sanitation Standard Operating Procedures): Step-by-step cleaning instructions.
- Control of Operations
- [ ] Documented Procedure and Records for Receipt, Storage, Loading, Unloading and Transport
- [ ] Documented Procedure and Records for High-Risk Processes
- [ ] Documented Procedure and Records for the Prevention and Removal of Foreign Objects.
- [ ] Documented Procedure and Records for Waste Disposal
- [ ] Environmental Monitoring Program (EMP): Swabbing schedules, methods and results for Indicator Organisms and Listeria or Salmonella.


Last Steps to ensuring your SQF System is Audit Ready
You need to demonstrate that your SQF food safety management system is effective. This is achieved by Validation and Verification that your SQF System meets the requirements of the SQF Food Safety Code. A final subsequent Senior Management Review helps tick the boxes to ensure that you truly are ready for that SQF Certification Audit.
SQF Edition 9 Checklist – Last Steps to ensuring your SQF System is Audit Ready
SQF System Validation and Effectiveness (Mandatory) – Documented Procedure and Records that Validate:
[ ] Good Manufacturing Practices are in place and effective
[ ] Critical food safety limits are reviewed annually and when changes occur; and
[ ] Changes to the processes or procedures are assessed
Verification Activities (Mandatory)
[ ] Documented Schedule, Methods and Records for Verification of Food Safety Controls including GMP and Food Safety Plans
Management Review (Mandatory) – Documented Records of a SQF System Review by Senior Site Management including:
[ ] Changes to food safety management system documentation
[ ] Food safety culture performance
[ ] Food safety objectives and performance measures
[ ] Corrective and preventative actions and trends in findings from internal and external audits, customer complaints, and verification and validation activities
[ ] Hazard and Risk Management System

How to Guarantee an "Excellent" Audit Result
The most common reason for a failed or “C-rating” audit isn’t a lack of effort—it’s fragmented documentation. If your SOPs don’t match your logs, or your Risk Assessments are missing technical logic, the auditor will issue a “Major” non-conformity.
Our SQF Edition 9 Template Toolkit doesn’t just give you the files; it gives you the technical support to ensure they are implemented correctly.
Why our users pass with 95+ scores:
- Pre-Linked Framework: Our Module 2 and Module 11 documents are cross-referenced to ensure no gaps.
- Audit-Response Support: If an auditor asks for a revision, we help you write it.
- Practitioner-Approved: Developed by former auditors to speak the language of the SQF Code.
[Download Your Audit-Ready SQF Toolkit & Get Technical Support Until You’re Certified]
About the Author - Tony Connor Bio
After gaining an Honors Degree in Molecular Biology and Biochemistry at the highly-rated Durham University, Tony Connor embarked on a highly successful career in the food industry.
Tony was appointed Laboratory Manager in 1989, Technical Manager of the UK’s largest multi-product dairy facility in 1993 and qualified as a Lead Assessor in 1994.
Over the years, Tony has gained experience in a variety of roles in the Food Industry including leading roles in Processing, Production, Operations, Quality, New Product Development and Technical departments.
During his career, he has commissioned both new sites and brought old sites up to SQF certification standard, helping many SQF Practitioners achieve SQF food safety certification for their organization.
With over 35 years’ experience in the food industry, Tony is a highly-regarded food safety expert and provides food operators both food certification services and hygiene, HACCP and Auditor training.

