FSSC 22000 Version 6 Food Safety Management System Implementation Package for Food Manufacturers
TCI systems is pleased to announce the launch of our FSSC 22000 Certification Package for Food Manufacturers Version 6
This is an ideal package for Food Manufacturers looking to achieve certification to the internationally recognized FSSC 22000 Certification Scheme Version 6 which is based on the integration of procedures compliant with the ISO 22000:2018 food safety management system standard, Technical Specification TS ISO 22002 part 1 Prerequisite Programmes for Food Manufacturers and FSSC 22000 Certification Scheme Additional Requirements Version 6 published April 2023
This really is our most complete documentation, project implementation and training support system .... an all in one easy to use package.
The FSSC 22000 Certification Package includes:
- Food Safety Management System Procedures
- Prerequisite Programmes Manual
- Operational Prerequisite Programme Templates
- Food Safety Management System Records
- HACCP Manual including the FSSC 22000 HACCP Calculator
- A set of PowerPoint Training Presentations covering ISO 22000, GMPs, Prerequisites, HACCP and Internal Audits
- ISO 22000/22002/FSSC 22000 Requirements Gap Analysis Checklists
- New Version 6 Implementation Workbook to guide you through the implementation of your FSSC 22000 compliant Food Safety Management System
- Start Up Guide
- Allergen Management Module & Risk Assessment Tool
- Supplier Risk Assessment Tool
- Product Development Module
- Complaint Management Guidelines & Analyser
- Hygiene Inspection Training
- Verification Schedule Risk Assessment Tool and Template
- Food Fraud Prevention Procedures and Raw Material Food Fraud Assessment Tool
- Free online support via e-mail
Food Safety Management System
The Food Safety Manual contains comprehensive top level procedures templates that match the clauses of the ISO 22000:2018 standard and form the foundations of your Food Safety Management System so you don't have to spend 1,000's of hours writing compliant procedures.

Below is a table that shows how the documents match the requirements of the ISO 22000 standard with the Food Safety Management System provided to assist you in implementing the system and understanding the requirements of the standard.
| FSSC 22000 Food Safety & Quality Management System | |
|---|---|
| 4 Context of the organization | |
| FSMS 4.1 Understanding the organization and its context | |
| FSMS 4.2 Understanding the needs and expectations of interested parties | |
| FSMS 4.3 Determining the scope of the food safety & quality management system | |
| FSMS 4.4 Food safety & quality management system | |
| 5 Leadership | |
| FSMS 5.1 Leadership and commitment | |
| FSMS 5.1 Food safety & quality culture planning | |
| FSMS 5.2 Policy | |
| FSMS 5.3 Organizational roles, responsibilities and authorities | |
| 6 Planning | |
| FSMS 6.1 Actions to address risks and opportunities | |
| FSMS 6.2 Objectives of the food safety & quality management system and planning to achieve them | |
| FSMS 6.3 Planning of changes | |
| 7 Support | |
| FSMS 7 Support includes: | 7.1 Resources |
| 7.1.1 General | |
| 7.1.2 People | |
| 7.1.3 Infrastructure | |
| 7.1.4 Work environment | |
| 7.1.5 Externally developed elements of the food safety & quality management system | |
| 7.1.6 Control of externally provided processes, products or services | |
| 7.2 Competence | |
| 7.3 Awareness | |
| FSMS 7.4 Communication includes: | 7.4.1 General |
| 7.4.2 External communication | |
| 7.4.3 Internal communication | |
| FSMS 7.5 Documented information includes: | 7.5.1 General |
| 7.5.2 Creating and updating | |
| 7.5.3 Control of documented information | |
| 8 Operation | |
| FSMS 8.1 Operational planning and control | |
| FSMS 8.1 Product development module/folder | |
| FSMS 8.2 Prerequisite programmes (PRPs) | |
| FSMS 8.3 Traceability system | |
| FSMS 8.4 Emergency preparedness and response | |
| 8.5 Hazard control | |
| 8.5 Hazard control module/folder | |
| FSMS 8.5.1 Preliminary steps to enable hazard analysis | 8.5.1.1 General |
| 8.5.1.2 Characteristics of raw materials, ingredients and product contact materials | |
| 8.5.1.3 Characteristics of end products | |
| 8.5.1.4 Intended use | |
| 8.5.1.5 Flow diagrams and description of processes | |
| 8.5.1.5.1 Preparation of the flow diagrams | |
| 8.5.1.5.2 On-site confirmation of flow diagrams | |
| 8.5.1.5.3 Description of processes and process environment | |
| FSMS 8.5.2 Hazard analysis | 8.5.2.1 General |
| 8.5.2.2 Hazard identification and determination of acceptable levels | |
| 8.5.2.3 Hazard assessment | |
| 8.5.2.4 Selection and categorization of control measure(s) | |
| FSMS 8.5.3 Validation of control measure(s) and combinations of control measures | |
| FSMS 8.5.4 Hazard control plan (HACCP/OPRP plan) | 8.5.4.1 General |
| 8.5.4.2 Determination of critical limits and action criteria | |
| 8.5.4.3 Monitoring systems at CCPs and for OPRPs | |
| 8.5.4.4 Actions when critical limits or action criteria are not met | |
| 8.5.4.5 Implementation of the hazard control plan | |
| FSMS 8.6 Updating the information specifying the PRPs and the hazard control plan | |
| FSMS 8.7 Control of monitoring and measuring | |
| FSMS 8.8 Verification related to PRPs and the hazard control plan | 8.8.1 Verification |
| 8.8.2 Analysis of results of verification activities | |
| FSMS 8.9 Control of product and process nonconformities | 8.9.1 General |
| 8.9.2 Corrections | |
| 8.9.3 Corrective actions | |
| 8.9.4 Handling of potentially unsafe products | |
| 8.9.4.1 General | |
| 8.9.4.2 Evaluation for release | |
| 8.9.4.3 Disposition of nonconforming products | |
| FSMS 8.9.5 Withdrawal/recall | |
| 9 Performance evaluation | |
| FSMS 9.1 Monitoring, measurement, analysis and evaluation | 9.1.1 General |
| 9.1.2 Analysis and evaluation | |
| FSMS 9.2 Internal audit | |
| FSMS 9.3 Management review | 9.3.1 General |
| 9.3.2 Management review input | |
| 9.3.3 Management review output | |
| 10 Improvement | |
| FSMS 10 Improvement | 10.1 Nonconformity and corrective action |
| 10.2 Continual improvement | |
| 10.3 Update of the FSMS | |
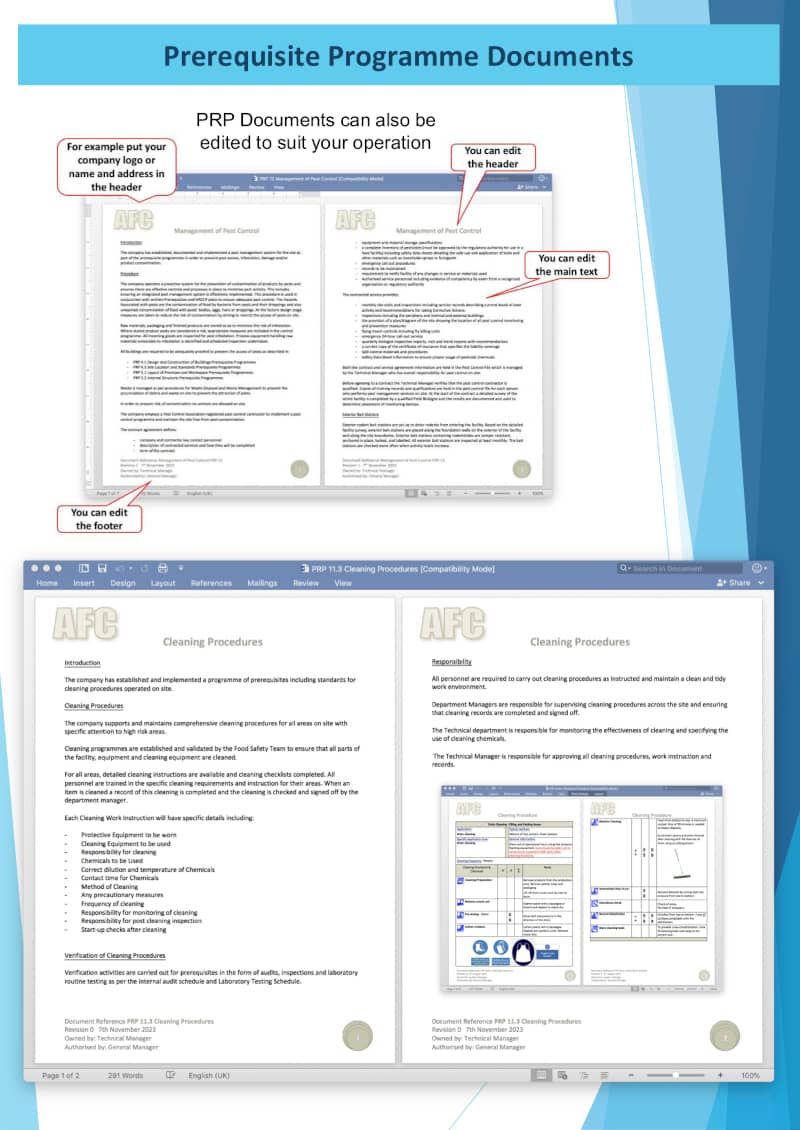
Prerequisite Programmes Manual
A comprehensive set of prerequisite programme templates compliant with TECHNICAL ISO/TS SPECIFICATION 22002-1 Prerequisite programmes on food safety — Part 1: Food manufacturing and FSSC 22000 Certification Scheme Additional Requirements Version 6 are included.

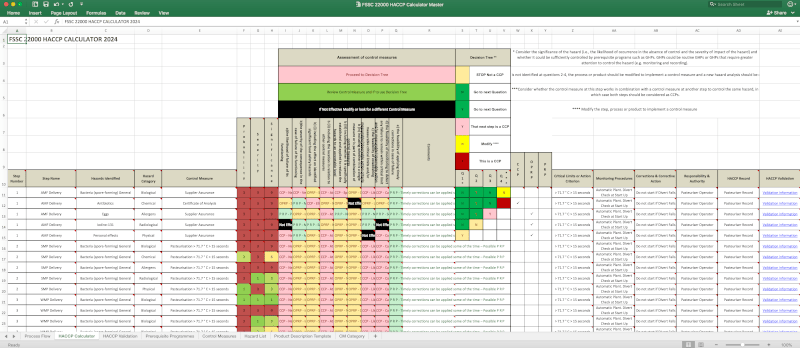
FSSC 22000 HACCP Calculator and Supplementary HACCP Documents
There are supplementary HACCP documents and tools including the FSSC 22000 HACCP Calculator & Instructions. This HACCP Calculator is based on the requirements of ISO 22000 and CODEX General Principles of Food Hygiene 2022 Edition HACCP System and Guidelines for its Application including a new 2022 Decision Tree.

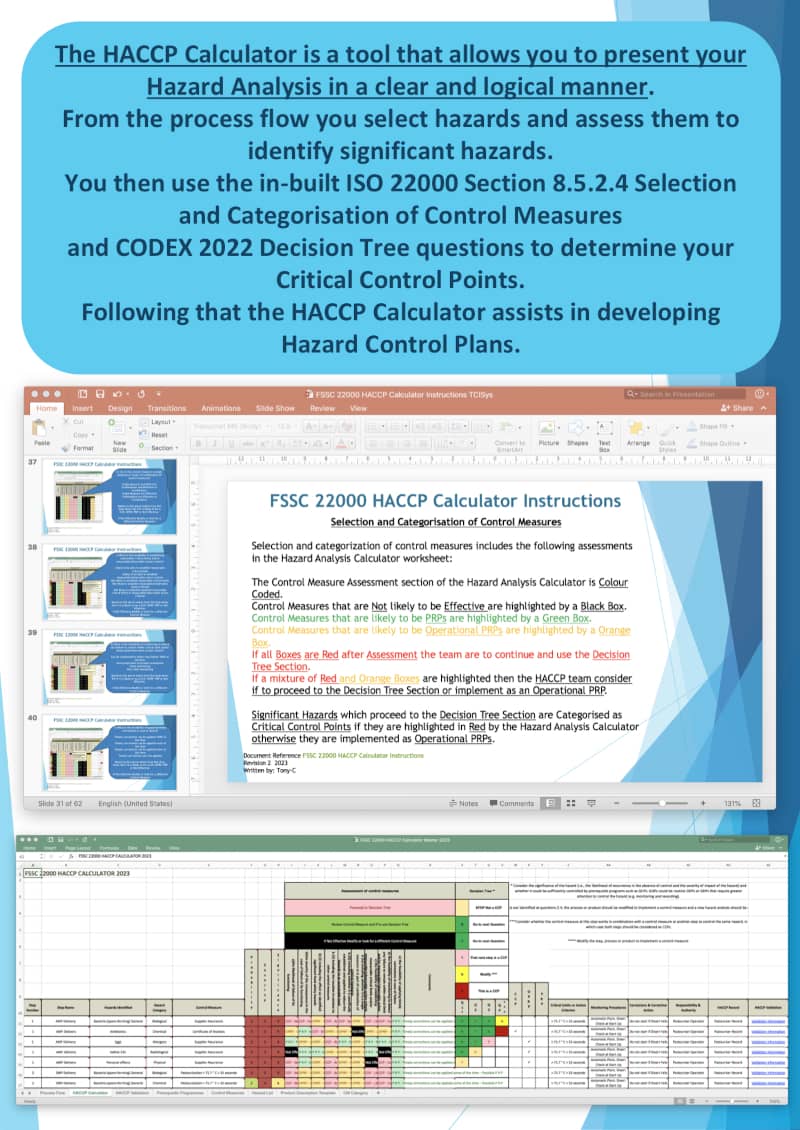
How does the FSSC 22000 HACCP Calculator help?
The ISO 22000 HACCP Calculator provides the Food Safety Team with a system to assess each of the control measures selected and formulating a Hazard Control Plan of Critical Control Points and Operational Prerequisite Programmes as per the new requirements in ISO 22000:2018 and the latest CODEX HACCP System Guidelines.
Operational Prerequisite Programmes Manual
A set of operational prerequisite programme samples with corresponding verification and validation records are provided.

FSQMS Record Templates
The package includes over 60 sample FSMS record templates.
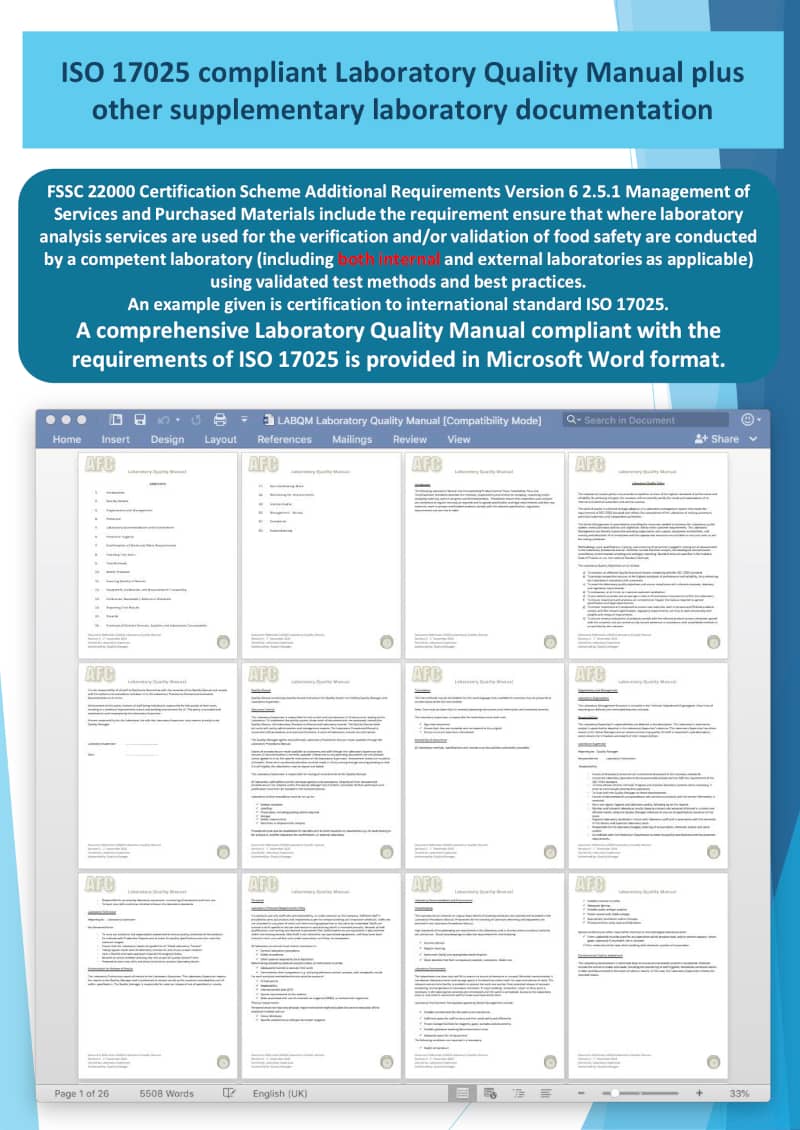
Laboratory Quality Manual
A comprehensive Laboratory Quality Manual based on the requirements of ISO 17025 is provided in Microsoft Word format. The laboratory quality manual includes template records, procedures and product sampling plans.
Training – 10 PowerPoint Presentations are included

Introduction to ISO 22000
This PowerPoint presentation will introduce the ISO 22000 standard to the management team and explain exactly how to start the process of implementing an ISO 22000 compliant Food Safety Management System.
Food Safety Team: ISO 22000 Implementation Guide
The Food Safety Team: ISO 22000 Implementation Guide PowerPoint presentation supplied with the system explains to the Food Safety Team their role in implementing an ISO 22000:2018 compliant Food Safety Management System.
ISO 22000 Document Requirement Guide
The ISO 22000 Document Requirement Guide PowerPoint presentation supplied explains to the Management Team the documentation required in an ISO 22000 compliant Food Safety Management System.
FSSC 22000 Additional Requirements Presentation
The FSSC 22000 Additional Requirements Version 6 PowerPoint presentation supplied explains to the Management Team the Additional Requirements of the FSSC 22000 Certification Scheme.
GMP & Prerequisite Training
GMP and ISO 22002 Prerequisites PowerPoint presentations are included.
HACCP Training
An illustrated PowerPoint HACCP training presentation is included.
Internal Auditor Training Guide ISO 22000 2018
A PowerPoint Internal Auditor training presentation is included and can be used to train your Internal Auditors.
Internal Audit Training - Warehouse Audit
An example of a Warehouse Audit is included.
Internal Audit Training - GMP Audit
A PowerPoint training presentation of a GMP Audit protocol is included.
Internal Audit Checklists
There is a checklist for each section of the ISO 22000 standard.
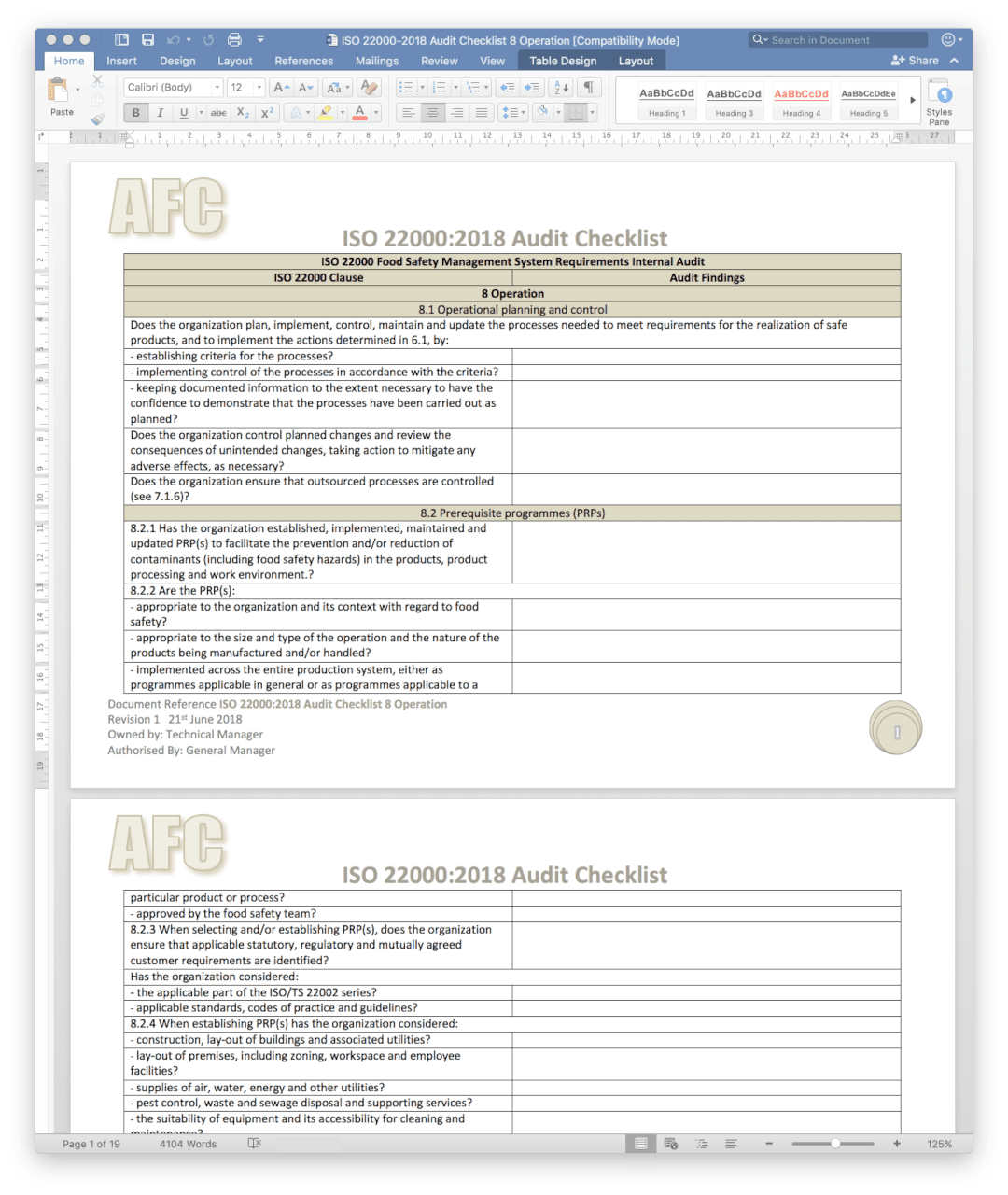
ISO 22000/22002 Audit Plan with Risk Rating
ISO 22000 & 22002 Audit Plans and risk rating system are included.
Project Tools
This contains project tools to assist in achieving ISO or FSSC 22000 certification.
Senior Management Implementation Guidance & Checklists
An 11 step Senior Management Implementation Checklist and Guidance are provided.
Project Plan
Excel and Word Project Planner templates are supplied with the system to help establish a Project Plan.
New Version 6 Comprehensive Implementation Workbook
This workbook is provided to guide you in the implementation of your Food Safety Management System. The workbook includes extensive Top Management and Food Safety Team Implementation Guidance.
FSSC 22000 Implementation Package Start Up Guide
We provide a Start Up Guide to help you navigate around the package and get to understand the contents.
Allergen Management Module & Risk Assessment Tool
Comprehensive allergen management documentation and the Allergen Management Tool which assist PRP 10.3 Allergen Control.
Food Fraud Prevention Procedures and Raw Material Food Fraud Assessment Tool
Product Development Module
Supplier Risk Assessment Tool
Complaint Management Guidelines & Analyser
Free online support via e-mail
We provide online support and expertise to assist you in developing your FSSC 22000 Food Safety Management System. Support is guaranteed until you achieve certification.
About the FSSC 22000 Certification Scheme
ISO 22000 Food safety management systems — Requirements for any organization in the food chain which was initially published in 2005 and has never been recognized by GFSI and GFSI recognition is playing an important role in Food Safety Management Systems Certification.
To address this in 2009, four leading global food manufacturers joined forces and created the FSSC 22000 scheme. By using the ISO 22000 standard, adding Prerequisite Program requirements based on ISO Technical Specifications/Publicly Available Standards (PAS) and FSSC 22000 additional requirements the Foundation created an ISO 22000 based scheme that was benchmarked and recognized by GFSI.
For example, under the FSSC 22000 Certification Scheme, ISO 22000:2018 Food safety management systems — Requirements for any organization in the food chain, ISO/TS 22002-1:2009 Prerequisite programmes on food safety — Part 1: Food manufacturing and FSSC 22000 Additional Requirements Version 5.1 are now applicable for the following Food Sector Categories:
- BIII Pre-process handling of plant products
- C0 Animal – Primary conversion
- CI Processing of perishable animal products
- CII Processing of perishable plant-based products
- CIII Processing of perishable animal and plant products (mixed products)
- CIV Processing of ambient stable products
- K Production of Bio-chemicals
The FSSC 22000 Scheme is now managed by Foundation FSSC 22000 and governed by an independent Board of Stakeholders, consisting of representatives from several sectors in the food industry. FSSC 22000 version 5.1 is compliant with the latest Global Food Safety Initiative (GFSI) Benchmarking Requirements - Version 2020. As of January 2024, there are 34,206 FSSC 22000 Certified Organizations.
In April 2023, the Foundation FSSC 22000 published Version 6 of its FSSC 22000 certification Scheme, it will be effective for audits from 1st April 2024. FSSC 22000-Quality certification has been removed in Version 6 but new additional requirements for quality are included in 2.5.8 Food Safety & Quality Culture and 2.5.9 Quality Control have been added.
Version 6 2.5 FSSC 22000 Additional Requirements
The following specific additional FSSC requirements for a food safety management system are included in the Scheme (All Food Chain Categories) unless stated:
- 2.5.1 Management of Services and Purchased Materials
- 2.5.2 Product Labelling and Printed Materials
- 2.5.3 Food Defense
- 2.5.4 Food Fraud Mitigation
- 2.5.5 Logo Use
- 2.5.6 Management of Allergens
- 2.5.7 Environmental Monitoring (Food Chain Categories BIII, C, I & K)
- 2.5.8 Food Safety and Quality Culture
- 2.5.9 Quality Control
- 2.5.10 Transport, Storage and Warehousing
- 2.5.11 Hazard Control and Measures for Preventing Cross-contamination (All excluding FII)
- 2.5.12 PRP Verification (Food Chain Categories BIII, C, D, G, I & K)
- 2.5.13 Product Design and Development (Food Chain Categories BIII, C, D, E, F, I & K)
- 2.5.14 Health Status (Food Chain Category D)
- 2.5.15 Equipment Management (All excluding FII)
- 2.5.16 Food Loss and Waste (All excluding I)
- 2.5.17 Communication Requirements
- 2.5.18 Requirements for Organization with Multi-Site Certification (Food Chain Categories A, E, F & G)
The following is an overview of the main changes for food manufacturers in 2.5 FSSC 22000 Additional Requirements Version 6
2.5.2 PRODUCT LABELING AND PRINTED MATERIALS (ALL FOOD CHAIN CATEGORIES)
Where a claim (e.g. allergen, nutritional, method of production, chain of custody, raw material status, etc.) is made on the product label or packaging, the organization shall maintain evidence of validation to support the claim and shall have verification systems in place.
2.5.3 FOOD DEFENSE (ALL FOOD CHAIN CATEGORIES)
a) Conduct and document the food defense threat assessment, based on a defined methodology, to identify and evaluate potential threats linked to the processes and products within the scope of the organization; and plans need to be documented based on the assessment.
2.5.4 FOOD FRAUD MITIGATION (ALL FOOD CHAIN CATEGORIES)
a) Conduct and document the food fraud vulnerability assessment, based on a defined methodology, to identify and assess potential vulnerabilities; and plans need to be documented based on the assessment.
2.5.6 MANAGEMENT OF ALLERGENS (ALL FOOD CHAIN CATEGORIES)
New requirements for a documented allergen management plan that includes:
- A list of all the allergens handled on site, including in raw materials and finished products;
- Identification and implementation of control measures to reduce or eliminate the risk of cross-contamination, based on the outcome of the risk assessment; and
- Validation and verification of these control measures shall be implemented and maintained as documented information.
- Precautionary or warning labels – limits of use.
- All personnel shall receive training in allergen awareness and specific training on allergen control measures associated with their area of work;
- The allergen management plan shall be reviewed at least annually, and following any significant change that impacts food safety, a public recall or a product withdrawal by the organization as a result of an allergen/s, or when trends in industry show contamination of similar products relating to allergens.
2.5.7 ENVIRONMENTAL MONITORING (FOOD CHAIN CATEGORIES BIII, C, I & K)
New requirements for:
- A risk-based environmental monitoring program for the relevant pathogens, spoilage, and indicator organisms
- Shall comply with legal and customer requirements.
- The environmental monitoring program shall be reviewed for continued effectiveness and suitability, at least annually, and more often if required, including when…
New 2.5.8 FOOD SAFETY AND QUALITY CULTURE (ALL FOOD CHAIN CATEGORIES)
New requirements for:
Commitment to cultivating a positive food safety and quality culture, including:
- Communication,
- Training,
- Employee feedback and engagement, and
- Performance measurement of defined activities covering all sections of the organization impacting on food safety and quality.
A requirement for objective(s) to be supported by a documented food safety and quality culture plan.
New 2.5.9 QUALITY CONTROL (ALL FOOD CHAIN CATEGORIES)
New requirements to establish, implement and maintain a quality policy and quality objectives.
New requirements to establish, implement and maintain quality parameters in line with finished product specifications and undertake analysis and evaluation of the results of the quality control parameters
New requirements to include quality elements as defined in clause 9.2 Internal audit within the scope of the internal audits.
New requirements to establish and implement quantity control procedures, including for unit, weight, and volume
New requirements to establish and implement line start-up and change-over procedures
2.5.10 TRANSPORT, STORAGE AND WAREHOUSING (ALL FOOD CHAIN CATEGORIES)
Transport combined with storage and warehousing
New requirements where transport tankers are used
2.5.11 HAZARD CONTROL AND MEASURES FOR PREVENTING CROSS- CONTAMINATION (ALL FOOD CHAIN CATEGORIES, EXCLUDING FII)
New requirements relating to foreign matter management, including a risk assessment in place to determine the need and type of foreign body detection equipment required including magnets, metal detectors, X-ray equipment, filters, and sieves.
2.5.13 PRODUCT DESIGN AND DEVELOPMENT (FOOD CHAIN CATEGORIES BIII, C, D, E, F, I & K)
New requirements for on-going shelf-life verification to be in place
New requirements for ready-to-cook products to have validated cooking instructions provided on the product label or packaging
2.5.15 EQUIPMENT MANAGEMENT (ALL FOOD CHAIN CATEGORIES, EXCLUDING FII)
New requirements for equipment specifications, a documented risk-based change management process for equipment changes or new equipment and evidence of successful commissioning
2.5.16 FOOD LOSS AND WASTE (ALL FOOD CHAIN CATEGORIES, EXCLUDING I)
New requirements for a documented policy and objectives detailing the organization’s strategy to reduce food loss and waste
New requirements to have controls in place to manage donated products
New requirements to manage surplus products or by-products intended as animal feed/food
New requirement for these activities to comply with the applicable legislation, be kept up to date, and not have a negative impact on food safety.
2.5.17 COMMUNICATION REQUIREMENTS (ALL FOOD CHAIN CATEGORIES)
New requirement for the notification of events or situations and to implement suitable measures as part of the emergency preparedness and response process.
See www.fssc22000.com for more details about FSSC 22000 Certification Scheme Version 6 | April 2023.
The following table shows where Version 6 2.5 FSSC 22000 Additional Requirements are covered in the Implementation Package Documentation
| FSSC 22000 Additional Requirements | FSSC 22000 FSQMS Documents |
|---|---|
|
2.5.1 MANAGEMENT OF SERVICES AND PURCHASED MATERIALS (ALL FOOD CHAIN CATEGORIES) |
|
| a) Laboratory Analysis Services - using validated test methods and best practices | LABQM Laboratory Quality Manual PRP 5.5 Laboratory Manual |
| b) Documented procedure for procurement in emergency situations | PRP 9.1 Purchasing Prerequisite Programmes PRP 9.2 Supplier Approval and Monitoring PRP 9.3 Control of Incoming Materials FSMS 7 Support |
| c) Policy for the procurement of animals, fish and seafood that are subject to control of prohibited substances (e.g., pharmaceuticals, veterinary medicines, heavy metals, and pesticides) | PRP 9.1 Purchasing Prerequisite Programmes PRP 9.2 Supplier Approval and Monitoring PRP 9.3 Control of Incoming Materials |
| d) Review process for raw material and finished product specifications to ensure continued compliance with food safety, quality, legal and customer requirements. | PRP 9.1 Purchasing Prerequisite Programmes PRP 9.2 Supplier Approval and Monitoring PRP 9.3 Control of Incoming Materials PRP 9.3A Incoming Material Specification Requirements FSMS 8.1 Operational planning and control |
| e) Packaging manufacturers need to establish criteria related to the use of recycled packaging as a raw material and ensure that relevant legal and customer requirements are being met. | |
|
2.5.2 PRODUCT LABELING AND PRINTED MATERIALS (ALL FOOD CHAIN CATEGORIES) |
|
| a) Finished products are labelled according to all applicable statutory and regulatory requirements in the country of intended sale, including allergen and customer specific requirements. | PRP 9.1 Purchasing Prerequisite Programmes PRP 9.2 Supplier Approval and Monitoring PRP 9.3 Control of Incoming Materials FSMS 7 Support |
| b) Where a product is unlabelled, all relevant product information shall be made available to ensure the safe use of the food by the customer or consumer. | |
| c) Where a claim (e.g. allergen, nutritional, method of production, chain of custody, raw material status, etc.) is made on the product label or packaging, the organization shall maintain evidence of validation to support the claim and shall have verification systems in place. | |
| d) For food chain category I, Packaging Materials artwork management and print control procedures shall be established and implemented. | |
|
2.5.3 FOOD DEFENSE (ALL FOOD CHAIN CATEGORIES) |
|
| 2.5.3.1 Threat Assessment | |
| a) Conduct and document the food defense threat assessment | PRP 18.1 Food Defence System PRP 18.2 Access Controls PRP 18 Food Threat Assessment & Mitigation Plan PRP 18 Food Defence Mitigation Strategies Checklists |
| b) Develop and implement appropriate mitigation measures for significant threats. | |
| 2.5.3.2 Plan | |
| a) Documented food defense plan, based on the threat assessment. | PRP 18.1 Food Defence System PRP 18.2 Access Controls PRP 18 Food Threat Assessment & Mitigation Plan Summary PRP 18 Food Defence Mitigation Strategies Checklists |
| b) The food defense plan shall be implemented and supported. | |
| c) The plan shall comply with applicable legislation, cover the processes and products and be kept up to date. | |
| d) For food chain category FII, in addition to the above, the organization shall ensure that their suppliers have a food defense plan in place. | |
|
2.5.4 FOOD FRAUD MITIGATION (ALL FOOD CHAIN CATEGORIES) |
|
| 2.5.4.1 Vulnerability Assessment | |
| a) Conduct and document the food fraud vulnerability assessment, based on a defined methodology. | PRP 9.4 Food Fraud Prevention PRP 9.4A Food Fraud Assessment Tool & Instructions PRP 9.4A Food Fraud Raw Material Assessment Tool |
| b) Develop and implement appropriate mitigation measures for significant vulnerabilities. | |
| 2.5.4.2 Plan | |
| a) The organization shall have a documented food fraud mitigation plan, based on the output of the vulnerability assessment. | PRP 9.4 Food Fraud Prevention PRP 9.4A Food Fraud Assessment Tool & Instructions PRP 9.4A Food Fraud Raw Material Assessment Tool |
| b) The food fraud mitigation plan shall be implemented and supported. | |
| c) The plan shall comply with the applicable legislation, cover the processes and products within the scope of the organization and be kept up to date. | |
| d) For food chain category FII, in addition to the above, the organization shall ensure that their suppliers have a food fraud mitigation plan in place. | |
|
2.5.5 LOGO USE (ALL FOOD CHAIN CATEGORIES) |
|
| Certified organizations are entitled to use the FSSC 22000 logo. The FSSC 22000 logo may be used on the organization’s printed matter, website and other promotional material but is subject to prescribed design specifications. |
|
|
2.5.6 MANAGEMENT OF ALLERGENS (ALL FOOD CHAIN CATEGORIES) |
|
| Requirements for a documented allergen management plan that includes: | |
| a) A list of all the allergens handled on site. | PRP 10.3 Allergen Control PRP 10.3 Allergen Management System Folder including: PRP 10.3 Comprehensive Allergen Management System Allergen Management Tool PRP 10.3A Allergen Clean Validation PRP 10.3B Allergen Clean Verification Allergen Warning Labels Allergen Information Forms |
| b) Risk assessment covering all potential sources of allergen cross-contamination. | |
| c) Identification and implementation of control measures. | |
| d) Validation and verification of these control measures. | |
| e) Precautionary or warning labels only used where the outcome of the risk assessment identifies allergen cross-contamination as a risk to the consumer, even though all the necessary control measures have been implemented. | |
| f) All personnel shall receive training in allergen awareness and specific training on allergen control measures associated with their area of work. | |
| g) The allergen management plan shall be reviewed at least annually, and following any significant change that impacts food safety etc. | |
| h) For Food Chain Category D: Where there is no allergen-related legislation for the country of sale pertaining to animal feed, this section of the Scheme requirements may be indicated as ‘Not Applicable,’ unless a claim relating to an allergen status has been made on the animal feed. | |
|
2.5.7 ENVIRONMENTAL MONITORING (FOOD CHAIN CATEGORIES BIII, C, I & K) |
|
| a) A risk-based environmental monitoring program for the relevant pathogens, spoilage, and indicator organisms. | PRP 11.5 Monitoring of Cleaning Effectiveness PRP 11.5A Environmental Monitoring Planning |
| b) Documented procedure for the evaluation of the effectiveness of all controls on preventing contamination from the manufacturing environment. | |
| c) Data of the environmental monitoring activities including regular trend analysis. | |
| d) The environmental monitoring program shall be reviewed for continued effectiveness and suitability, at least annually, and more often if required, including when the following triggers occur: …. | |
|
2.5.8 FOOD SAFETY AND QUALITY CULTURE (ALL FOOD CHAIN CATEGORIES) |
|
| a) In accordance with and in addition to clause 5.1 of ISO 22000:2018, as part of the organizations’ commitment to cultivating a positive food safety and quality culture. Senior management establish, implement and maintain a food safety and quality culture objective(s). The following elements addressed as a minimum: Communication, Training, Employee feedback and engagement, and Performance measurement of defined activities. |
FSMS 5.1 Leadership and commitment FSMS 5.1 Food Safety Culture Planning FSMS 5.1 FS Culture - Expected Behaviours FSMS 6.2 Food Safety & Quality Objectives |
| b) The objective(s) shall be supported by a documented food safety and quality culture plan, with targets and timelines and included in the management review and continuous improvement processes of the management system. | |
|
2.5.9 QUALITY CONTROL (ALL FOOD CHAIN CATEGORIES) |
|
| a) The organization shall: In addition to, and aligned with, clauses 5.2 and 6.2 of ISO 22000:2018, establish, implement and maintain a quality policy and quality objectives. Establish, implement and maintain quality parameters in line with finished product specifications, for all products and/or product groups within the scope of certification, including product release that addresses quality control and testing. In addition to, and aligned with, clauses 9.1 and 9.3 of ISO 22000:2018, undertake analysis and evaluation of the results of the quality control parameters, as defined above, and include it as an input for the management review; and In addition to, and aligned with, clause 9.2 of ISO 22000:2018, include quality elements as defined in this clause, within the scope of the internal audit. |
FSMS 5.2 Food Safety & Quality Policy FSMS 6.2 Food Safety & Quality Objectives Product Specifications FSMS 9.2 Internal Audits & Inspections ISO 22000 and ISO 22002 Audit Plan with Risk Rating |
| b) Quantity control procedures, including for unit, weight, and volume, shall be established, and implemented, to ensure products meet the applicable customer and legal requirements. This shall include a program for calibration and verification of equipment used for quality and quantity control. | FSMS 8.1 Operational planning and control FSMS 8.7 Control of monitoring and measuring FSMS 9.1 Monitoring, measurement, analysis and evaluation PRP 17.2 Product Labelling Controls |
| c) Line start-up and change-over procedures shall be established and implemented to ensure products, including packaging and labelling, meet applicable customer and legal requirements. This shall include having controls in place to ensure labelling and packaging from the previous run have been removed from the line. | |
|
2.5.10 TRANSPORT, STORAGE AND WAREHOUSING (ALL FOOD CHAIN CATEGORIES) |
|
| a) A requirement to establish, implement, and maintain a procedure and specified stock rotation system that includes FEFO (First Expired, First Out) principles in conjunction with the FIFO (First In, First Out) requirements. | PRP 16.1 Warehousing Prerequisites PRP 16.2 Warehousing Procedures PRP 16.3 Dispatch and Distribution Prerequisites PRP 16.3 Appendix - Dispatch and Distribution Procedure PRP 9.3 Control of Incoming Materials |
| b) For food chain category C0, in addition to ISO/TS 22002-1:2009 clause 16.2 Warehousing requirements, where applicable, there needs to specified requirements in place that define post-slaughter time and temperature for chilling or freezing of the products. | |
| c) For food chain category FI Retail/Wholesale/E-commerce, in addition to BSI/PAS 221:2013 clause 9.3, the organization shall ensure that product is transported and delivered under conditions which minimize the potential for contamination. | |
| d) Where transport tankers are used, the following shall apply in addition to clause 8.2.4 of ISO 22000:2018: etc. | |
|
2.5.11 HAZARD CONTROL AND MEASURES FOR PREVENTING CROSS- CONTAMINATION (ALL FOOD CHAIN CATEGORIES, EXCLUDING FII) |
|
| a) For food chain categories BIII, C and I, Production of food packaging and packaging materials, there are SPECIFIC requirements for packaging used to impart or provide a functional effect on food such as shelf life extension. | FSMS 8.1 Operational Planning and Control PRP 2 HACCP Prerequisite Programmes PRP 10.1 Prevention of Contamination PRP 10.4 Prevention of Physical Contamination FSMS 8.5.2 Hazard Analysis Operational Prerequisite Programmes to prevent foreign body contamination: OPRP 1 Hygiene Policy OPRP 2 Glass Policy OPRP 3 Ingredients Foreign Body Control Policy OPRP 4 Metal Detection OPRP 5 Nut Handling Procedure OPRP 6 Control of Knives OPRP 7 Control of Brittle Materials OPRP 8 Glass & Brittle Material Breakage Procedure OPRP 9 Control of First Aid Dressings |
| b) For food chain category C0, Processing of perishable animal products, in addition to ISO/TS 22002- 1:2009 10 Measures for prevention of cross-contamination clause 10.1 General requirements there needs to be specified requirements for an inspection process at lairage and/or at evisceration to ensure animals are fit for human consumption. | |
| c) For food chain category D Animal Feed Production, the following requirement applies in addition to ISO/TS 22002- 6:2016 clause 4.7: The organization shall have in place procedures to manage the use of ingredients/additives that contain nutrients components that can have an adverse animal health impact. | |
| d) For all food chain categories, excluding FII, the following requirements relating to foreign matter management apply, in addition to clause 8.2.4 (h) of ISO 22000:2018: The organization shall have a risk assessment in place to determine the need and type of foreign body detection equipment required. Where the organization deems no foreign body detection equipment is necessary, justification shall be maintained as documented information. Foreign body detection equipment includes equipment such as magnets, metal detectors, X-ray equipment, filters, and sieves. A documented procedure shall be in place for the management and use of the equipment selected. The organization shall have controls in place for foreign matter management including procedures for the management of all breakages linked to potential physical contamination (e.g., metal, ceramic, hard plastic). |
|
|
2.5.12 PRP VERFITICATION (FOOD CHAIN CATEGORIES BIII, C, D, G, I & K) |
|
| The following additional requirement applies to ISO 22000:2018 clause 8.8.1: The organization shall establish, implement, and maintain routine (e.g., monthly) site inspections/PRP checks to verify that the site (internal and external), production environment and processing equipment are maintained in a suitable condition to ensure food safety… | FSMS 9.2 Internal Audits & Inspections FSMS 9.2 Plans & Checklists Folder PRP Verification Records Audit and Inspection Training Presentations, Forms and Documents |
|
2.5.13 PRODUCT DESIGN AND DEVELOPMENT (FOOD CHAIN CATEGORIES BIII, C, D, E, F, I & K) |
|
| A product design and development procedure shall be established, implemented, and maintained for new products and changes to product or manufacturing processes to ensure safe and legal products are produced. This shall include the following: | |
| Evaluation of the impact of the change on the FSMS taking into account any new food safety hazards (incl. allergens) introduced and updating the hazard analysis accordingly, | FSMS 8.1 Operational Planning and Control FSMS 8.1 Product Development Folder – NPD Documents including NPD 001 Product Development Plan FSR Product Change Approval Records |
| Consideration of the impact on the process flow for the new product and existing products and processes, | |
| Resource and training needs. | |
| Equipment and maintenance requirements. | |
| The need to conduct production and shelf-life trials to validate product formulation and processes are capable of producing a safe product and meet customer requirements. | |
| A process for on-going shelf-life verification shall be in place, at a frequency based on risk. | |
| New requirements for ready-to-cook products to have validated cooking instructions provided on the product label or packaging. | |
|
2.5.14 HEALTH STATUS (FOOD CHAIN CATEGORY D) |
|
| In addition to ISO/TS 22002-6 clause 4.10.1, the organization shall have a procedure to ensure that the health of personnel does not have an adverse effect on the feed production operations. Subject to legal restrictions in the country of operation, employees shall undergo a medical screening prior to employment in feed contact operations, unless documented hazards or medical assessment indicates otherwise. Additional medical examinations, where permitted, shall be carried out as required and at intervals defined by the organization. | |
|
2.5.15 EQUIPMENT MANAGEMENT (ALL FOOD CHAIN CATEGORIES, EXCLUDING FII) |
|
| Documented purchase specification in place, which addresses hygienic design, applicable legal and customer requirements, and the intended use of the equipment, including product handled. | FSMS 8.1 Operational Planning and Control PRP 8.1 Equipment Prerequisite Programmes PRPs PRP 8.2 Equipment Hygienic Design FSR Equipment Commissioning Checklist FSR Process Change Approval PRP 5.4 Equipment Design and Location |
| Risk-based change management process for new equipment and/or any changes to existing equipment, which shall be adequately documented including evidence of successful commissioning. | |
|
2.5.16 FOOD LOSS AND WASTE (ALL FOOD CHAIN CATEGORIES, EXCLUDING I) |
|
| In addition to clause 8 of ISO 22000:2018, the organization shall: | |
| a) Have a documented policy and objectives detailing the organization’s strategy to reduce food loss and waste within their organization and the related supply chain. | PRP 7 Waste Management Overview including FLW PRP 7.1 Waste Management PRPs PRP 7.5 Management of Surplus Food and Products for Animal Feed PRP 7 Food Loss and Waste Analysis |
| b) Have controls in place to manage products donated to not-for-profit organizations, employees, and other organizations; and ensure that these products are safe to consume. | |
| c) Manage surplus products or by-products intended as animal feed/food to prevent contamination of these products. | |
| d) These processes shall comply with the applicable legislation, be kept up to date, and not have a negative impact on food safety. | |
|
2.5.17 COMMUNICATION REQUIREMENTS (ALL FOOD CHAIN CATEGORIES) |
|
| In addition to clause 8.4.2 of ISO 22000:2018, the organization shall inform the certification body within 3 working days of the commencement of the events or situations below and implement suitable measures as part of their emergency preparedness and response process: | |
| a) Serious events that impact the FSMS, legality and/or the integrity of the certification including situations that pose a threat to food safety, or certification integrity as a result of a Force majeure, natural or man-made disasters (e.g., war, strike, terrorism, crime, flood, earthquake, malicious computer hacking, etc.); | FSMS 7.4 Communication FSMS 8.4 Emergency preparedness and response FSMS 8.9.5 Withdrawal/recall |
| b) Serious situations where the integrity of the certification is at risk and/or where the Foundation can be brought into disrepute. These include, but are not limited to: Public food safety events (e.g., public recalls, withdrawals, calamities, food safety outbreaks, etc.) Actions imposed by regulatory authorities as a result of a food safety issue(s) where additional monitoring or forced shutdown of production is required. Legal proceedings, prosecutions, malpractice, and negligence. Fraudulent activities and corruption. |
|
|
2.5.18 REQUIREMENTS FOR ORGANIZATION WITH MULTI-SITE CERTIFICATION (FOOD CHAIN CATEGORIES E, F & G) |
|
| 2.5.15.1 – Central function Requirements for the management of the central function to ensure that sufficient resources are available, and that roles, responsibilities and requirements are clearly defined. |
|
| 2.5.15.2 - Internal Audit Requirements An internal audit procedure and program shall be established by the central function covering the management system, central function, and all sites. |
|


